Volume 13, Issue 4 (July & August: Special Issue on Cognitive Sciences 2022)
BCN 2022, 13(4): 531-550 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hoodgar M, Khosrowabadi R, Navi K, Mahdipour E. Brain Functional Connectivity Changes During Learning of Time Discrimination. BCN 2022; 13 (4) :531-550
URL: http://bcn.iums.ac.ir/article-1-2403-en.html
URL: http://bcn.iums.ac.ir/article-1-2403-en.html
1- Department of Computer Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Keywords: Electroencephalography (EEG), Functional connectivity, Time perception, Interval discrimination, Phase lag index
Full-Text [PDF 7275 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
According to various documents, physical senses are processed in specific areas of the brain, but no specific area of the brain processes the sense of internal time (Azari, Mioni, Rousseau, & Grondin, 2020; Matthews & Meck, 1991; Meck, 2006; Mioni, Grondin, Bardi, & Stablum, 2020; Nani et al., 2019; Tamburro, di Fronso, Robazza, Bertollo, & Comani, 2020; Umbach et al., 2020; Wiener, Turkeltaub, & Coslett, 2010; Wu, Shankar Gupta, Banerjee, Roy, & Piras, 2020). Perception of time, which is one of the most vital functions of the brain (internal experience of time), is an abstract concept that is created based on the information recorded in the brain (internal and external senses in addition to memory) with the help of a network of its regions. Of course, each region may refer to a different aspect of time (Buzsáki & Llinás, 2017; Coull & Droit-Volet, 2018; Gili, Ciullo, & Spalletta, 2018; Matthews & Meck, 2016; Nani et al., 2019).
In related research that has been done so far, potential mechanisms related to a diverse set of brain regions have been discovered, some of which are as follows: pre-supplementary motor regions (Gámez, Mendoza, Prado, Betancourt, & Merchant, 2019; Merchant et al., 2015; Protopapa et al., 2019), parietal regions (Bueti & Walsh, 2009; Hayashi, van der Zwaag, Bueti, & Kanai, 2018), insula (Wittmann, Simmons, Aron, & Paulus, 2010), midbrain dopaminergic neurons (Martel & Apicella, 2021; Soares, Atallah, & Paton, 2016), hippocampus and entorhinal cortex (Montchal, Reagh, & Yassa, 2019; Sugar & Moser, 2019; Umbach et al., 2020).
Therefore, the study and discovery of networks related to temporal perception are among the fascinating topics in the field of brain behavior that researchers have always attended. On the other hand, it is observed in many human experiences (such as learning music) that a person can better understand the accuracy of temporal events through practice (Chauvigné, Gitau, & Brown, 2014; Drayton & Furman, 2018; Siman-Tov et al., 2019). This diversity of neural areas may be related to human perception of time. Time perception reflects the nervous system’s dynamics because these dynamics result from the objective clock (Lad, Hurley, & Taber, 2020).
One of the primary factors in learning is the creation of new neural connections in the brain; these connections are created when the brain is engaged in a learning process. Many experimental results show that active interaction is the background of changes in the brain. That is, learning will not occur unless the brain is involved in an active engagement; for example, listening to a lecture or giving a presentation will never lead to permanent learning. The things that enhance training include facilitation, simulation, play, and role-playing that stimulate active interaction.
One of the criteria that show the effect of changes in the brain regions well is the functional connectivity (FC) criteria which shows the significant changes in the connection of different parts of the brain during the learning process (Shalbaf, Behnam, Sleigh, Steyn-Ross, & Steyn-Ross, 2015; Zippo, Della Rosa, Castiglioni, & Biella, 2018). On the other hand, electroencephalography (EEG) is a helpful tool for analyzing how to understand cognitive time intervals (inms). The combination of these two tools, namely functional connectivity, and EEG, can provide valuable information on how to record and form connections of parts related to the learning process of differentiation of time intervals (Gonzalez-Castillo & Bandettini, 2018). However, EEG acquisition suffers from the problem of volume conduction caused by the propagation of electric fields generated by a mainstream source to all (or more) of the surrounding sensors located on the scalp (Rouzbahani & Motie, 2017; Gonzalez-Castillo & Bandettini, 2018). This problem is a linear behavior. In such a case, standard FC estimation methods, such as coherence or cross-information, lead to the identification of functional connections that do not reflect actual interregional interactions in the brain (Imperatori et al., 2019).
Alternative methods can determine the functional connections and solve this problem. These methods include weighted phase lag index (WPLI) and phase lag index (PLI), which have been widely used in many studies to estimate the functional connectivity of EEG. Based on the obtained results, these two methods identify relative changes in the functional connectivity of the brain in conditions associated with changes in the level of consciousness (Gonzalez- Castillo & Bandettini, 2018).
The phase lag index was introduced by Stam, Nolte, & Daffertshofer (2007). Its main idea is not to pay attention to the phase lock, which is about the 0-axis phase difference. This criterion eliminates the effects of volume transfer (there is a risk of ignoring the actual immediate interactions). PLI can be the phase difference ratio between signals in multiple experiments above or below the zero-degree line (i.e., the true axis WPLI calculates phase synchronization, which is mainly used for linear interactions but also seems suitable for nonlinear coupling. The values of both indicators are in the range of 0 to one. Unlike PLI, WPLI weights the cross-spectrum according to the magnitude of the imaginary component. This issue allows it to limit the influence of cross-spectrum elements around the real axes, which risk changing their “true” sign with small noise perturbations. Such an index of phase synchronization was proposed by Vinck et al. This index is based only on the imaginary component of the cross-spectrum, which implies robustness to noise because uncorrelated noise sources will cause an increase in signal power. It has been shown that WPLI outperforms PLI, coherence, and imaginary coherence (IC) with real, local field potentials (LFP) data (Vinck et al., 2011).
As mentioned, the issue of time perception is a vital challenge that human beings face in various aspects of their lives. Researchers have constantly been challenged in calculating it and understanding its mechanism for each individual (Chen, Shore, Lewis, & Maurer, 2016; Grondin, 2010; Powers, Hillock-Dunn, & Wallace, 2016; Villalonga, Sussman, & Sekuler, 2021). In the present study, which is based on temporal perception, by comparing the timing of auditory stimuli (Azari et al., 2020; Ghaderi et al., 2018), we seek to show the functional relationships of neural network formation related to learning temporal perception. We aimed to understand how the hidden information of auditory stimuli (time intervals) is encoded in the content of brain signals. For this purpose, an intervention model with the help of acoustic stimuli with a distance of less than one second (short) and one second (long) was designed to study the learning process of temporal differentiation in healthy individuals. Experimental findings showed an overall improvement in the ability to store and compare learning stimuli in all target group members.
2. Materials and Methods
Ethical consideration
Ethical considerations of our study method have been approved by the Ethics Review Committee of Shahid Beheshti University. It is noteworthy that all stages of study and development methods have been approved and written by the Ethics Committee at the relevant university with the reference code I.R.SBU.REC.1399.040. The complete scenario of this research was explained to each participant, who voluntarily signed the relevant form and expressed their consent to participate in this test. These individuals had no previous information about assigning discrimination time intervals and have not been paid.
Study participants
Participants in this study were selected from undergraduate students in Medical Engineering, Computer Engineering, and Nursing at the Dezful Branch, Islamic Azad University, Iran. The total number of participants is 16 healthy right-handed volunteers, including 11 men and 5 women aged 22±1.7 years. Participants were tested before the preliminary test to ensure their ability to participate in the test. For this purpose, IQ (Penrose & Raven, 1936), depression, anxiety, and stress test (DASS42) (Brown, Chorpita, Korotitsch, & Barlow, 1997) and Edinburgh handedness test (Oldfield, 1971) were taken.
To assess the physical and mental condition of the individuals, they were asked to provide medical records, according to which participants had no hearing, memory, neurological impairment, pain status, or medication use that affected EEG data collection.
Before data collection, all the participants were asked not to consume alcohol or other beverages that contain caffeine which could affect the EEG data for 48h. However, two participants were excluded from the group due to their inability to learn the test. Also, one of the participants withdrew from the test for personal reasons.
Study materials
Participants cooperated in three tests: behavioral, cognitive, and neurophysiological. According to the scenario shown in Figure 1, the participants had to take one day for a preliminary test, including the Raven IQ test, the Edinburgh hand superiority test, and the DASS42. This work aimed to determine the initial status of the parameters that were thought to affect the participants’ learning process. The integrated visual and auditory (IVA) (Tinius, 2003) and N-back (Kane, Conway, Miura, & Colflesh, 2007) tests were also used to assess attention and working memory performance. Then, they conducted daily training sessions for 5 consecutive working days (except weekends). The experiments were performed in a room with controlled light and temperature without electrical interference. People were taught to focus only on auditory stimuli when sitting comfortably and without extra movement. In addition, they were asked not to think about anything else.
In the behavioral test, two time intervals were used simultaneously to prevent the participants’ mental adaptation to the test process to be a tool to test their perception from a neurological point of view. Participants were asked to identify standard and comparative intervals while focusing on the frequency of the sound stimulus. A modified scenario was used to determine the mechanism associated with learning time discrimination (Karmarkar & Buonomano, 2003). This stimulation protocol was used for all daily experiments, which general structure can be seen in Figure 1.
.jpg)
Study methods
In the first round of the work, EEG data (sampling frequency 256Hz) were recorded from the subject for 2 min with eyes closed and 2 min with eyes open without any stimulation. In this test, the electrode impedance was less than 10kΩ. Immediately after this stage, with the announcement of a short beep (1ms), the first round of trials was played for the participant, and the EEG signal was collected simultaneously. The trials were played similarly at the end of the first round and after a one-minute break in the second block. In addition, the same discipline was performed at the end of the third round after a one-minute break.
In each test round for the subject, stimuli consisting of two sounds with specific frequencies (4kHz and 1kHz) were played. Each pair of time intervals (standard and comparative) related to sound stimuli were separated by a fixed time interval (T) (Figure 1a and 1b). To avoid subjective adaptation, the fixed time intervals (T) of these pairs of intervals were randomly selected from the set (500, 600, or 700ms) for the short distance and (700,800 or 900ms) for the long distance.
Participants on the first day of the study could listen to the aforementioned audio structure in any number. Each trial consisted of playing test stimuli consisting of a pair of sounds shorter or longer than a pair of sounds with a standard interval (target stimulus) that generally had a duration equal to (T±Δt) (Figure 1). People were instructed to determine if the first or second interval was shorter or longer than the standard interval after hearing the sound stimuli through headphones by pressing one of the two keys on the test table. The tones were 15ms with a 5 on/off slope. The experiments were set up in such a way that on the first day, people were trained in cognitive, behavioral, and neuromarkers tests, and in the next five working days, in parallel with the learning process, the neuro markers, and cognitive tests were recorded
This training process was performed in four blocks of 40 trials with experimental stimuli adjusted for a long time randomly and intricately in each block. The method was used to determine the threshold values (Wright, Buonomano, Mahncke, & Merzenich, 1997). During each test block, an adaptive function reduces the comparison interval by as much as Δt after three correct answers but increases by the same amount of Δt for each incorrect answer. The Δt values were recorded at pivot points (decrease to increase or vice versa). Since the stimuli in the protocols had spaces equal to T±δt, the threshold is twice the mean of the total inverse values except for the first three trials. The effective learning curve is determined by linear orthogonal trend analysis (Bruning & Kintz, 1987).
Data acquisition and analysis
In this study, 16 electrodes were used to obtain EEG signals, which were arranged according to the international standard 10-10 (FP1, FP2, F5, F6, T7, C3, CZ, C4, T8, TP7, TP8, P3, P.Z., P4, O1, and O2). The sampling rate was set at 256 samples per second. Brain signals were recorded on the first and last day of the experiments to determine learning effects. With the help of PLI and WPLI, neural networks related to functional connectivity of different brain parts involved in learning time perception were investigated. In other words, brain areas associated with this process were examined to demonstrate the effect of time learning discrimination on neural activity.
Cognitive indicators included IQ, stress, depression, and anxiety. In addition, to assess a person’s level of auditory attention before the behavioral test, the IVA test (focusing on speed and alertness) was performed at the beginning of all training sessions. Standard and comparative time difference parameters were used to analyze the behavioral data of the test sessions. After the behavioral test, the N-back test (levels one and three) was performed to investigate the effect of temporal perception learning on working memory performance.
To prepare the obtained EEG signal, preprocessing operations were performed using EEGLAB toolbox version 14.1.2b (Delorme & Makeig, 2004) in MATLAB 2016b software, including bandpass filtering 1-40 Hz, trials segmentation, removing eye movement/blinking, and muscular artifact using independent component analysis approach and removing still noisy segments.
Then, to separate EEG data in ordinary frequency bands such as delta [1-4 Hz], theta [4-8 Hz], alpha [8-13 Hz], beta [30-30 Hz], gamma [30-40 Hz] as well as the calculation and analysis of the PLI and WPLI, the HERMES Toolbox (Niso et al., 2013) was utilized. Since EEG data were recorded only on the first and last day of the experiment, the effect of time interval discrimination learning on the EEG was assessed using the paired t-test.
3. Results
The results of cognitive and behavioral tests are reported in Table 1.
.jpg)
The Δt index depends on the short- and long-time intervals, which shows behavioral changes for the under-training group. Figure 2 shows the group learning chart for short and long periods.
.jpg)
PLI and WPLI were used to determine the status of functional connections. A statistical test was used to determine the changes in functional connections between the first and sixth days of the test. Data based on each channel and the delta, theta, alpha, beta, and gamma bands of brain signals were examined separately. The results of the tests performed can be seen in Figures 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22.
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
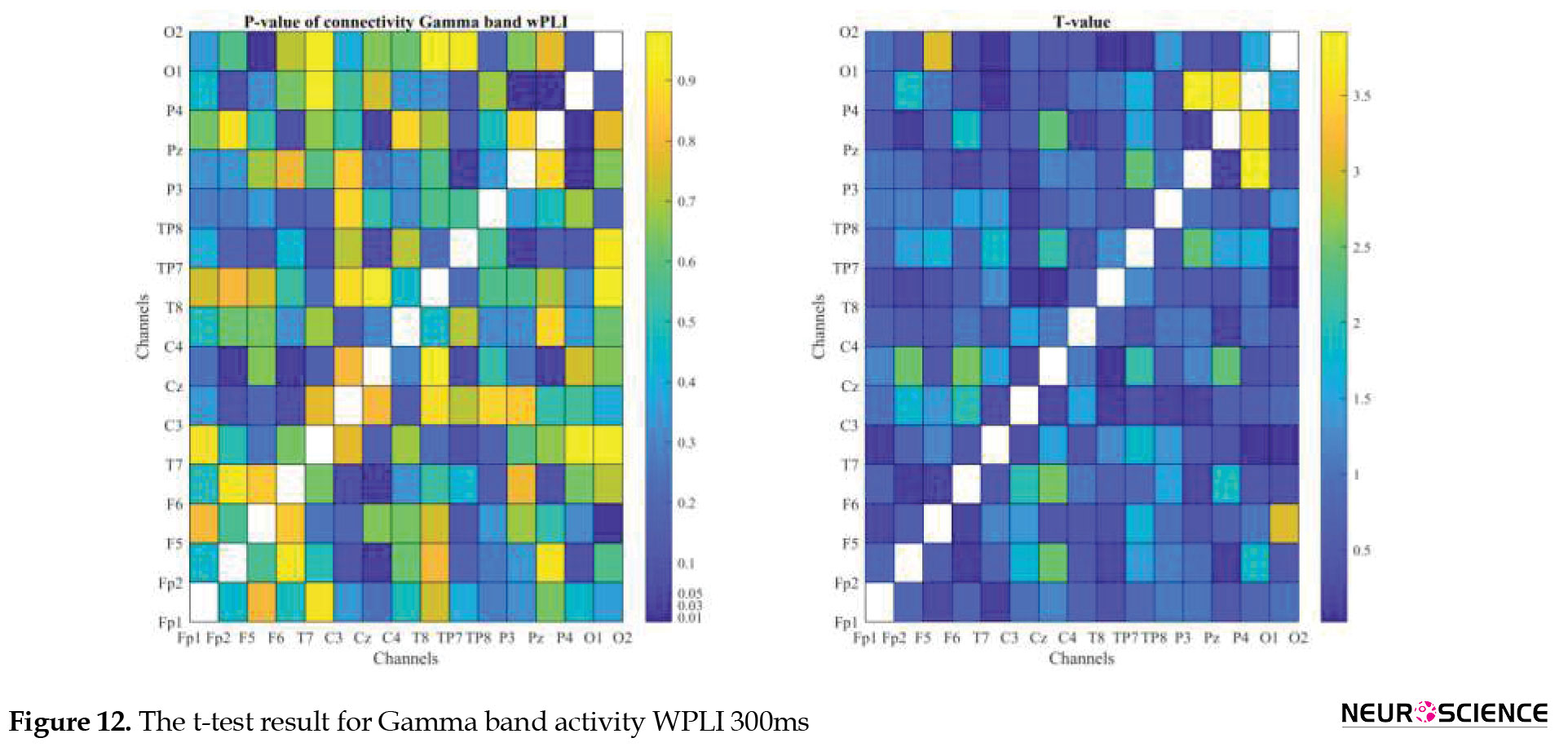
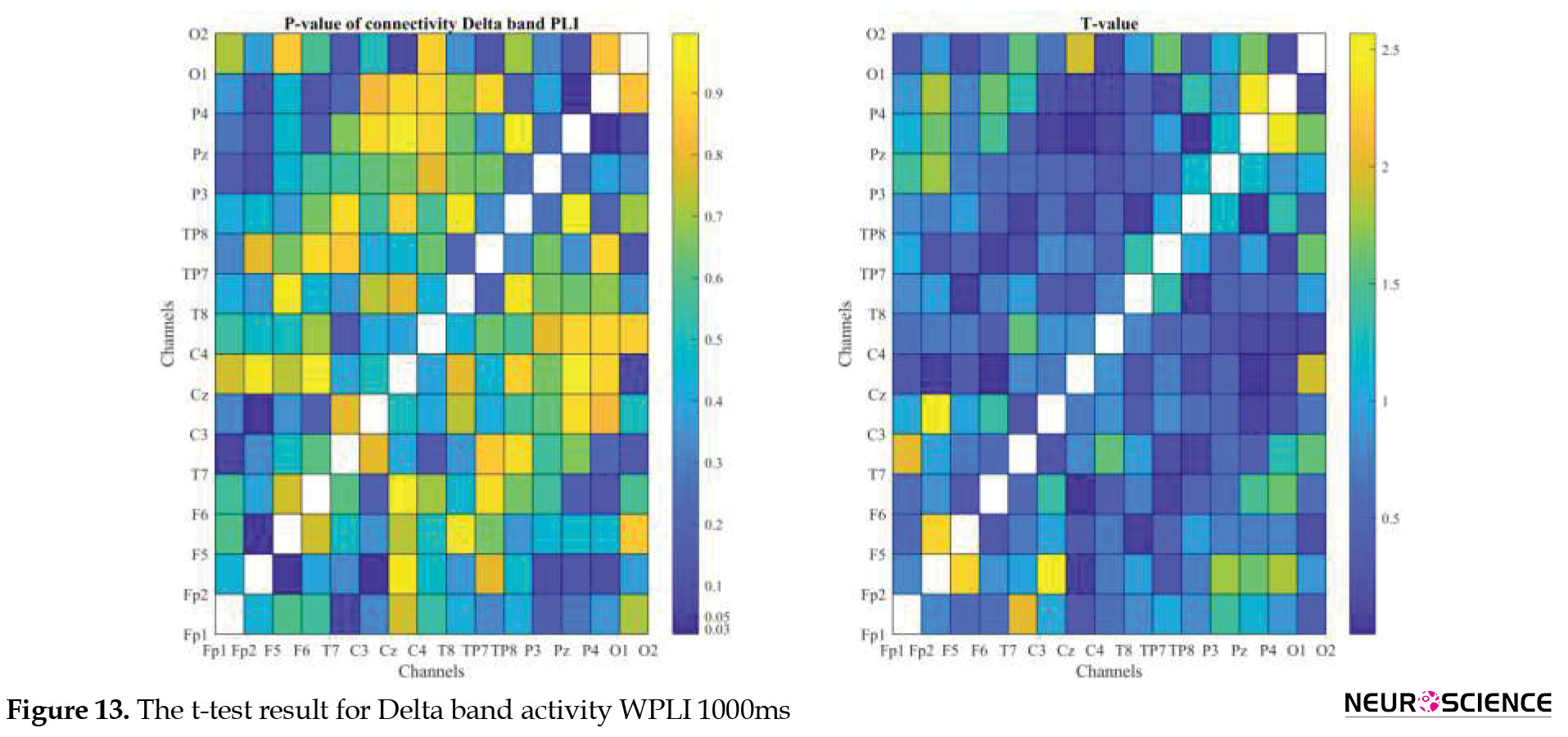

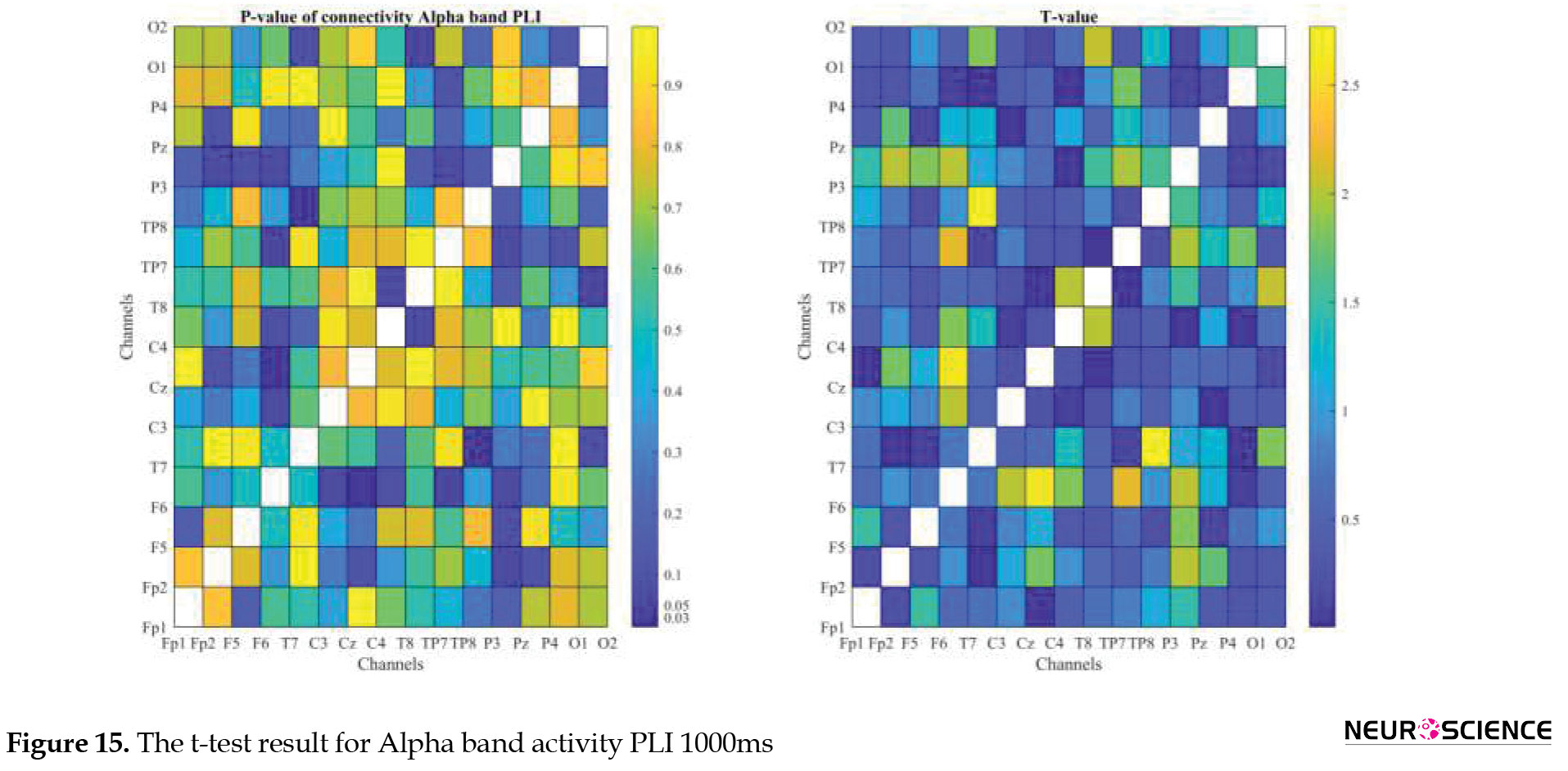
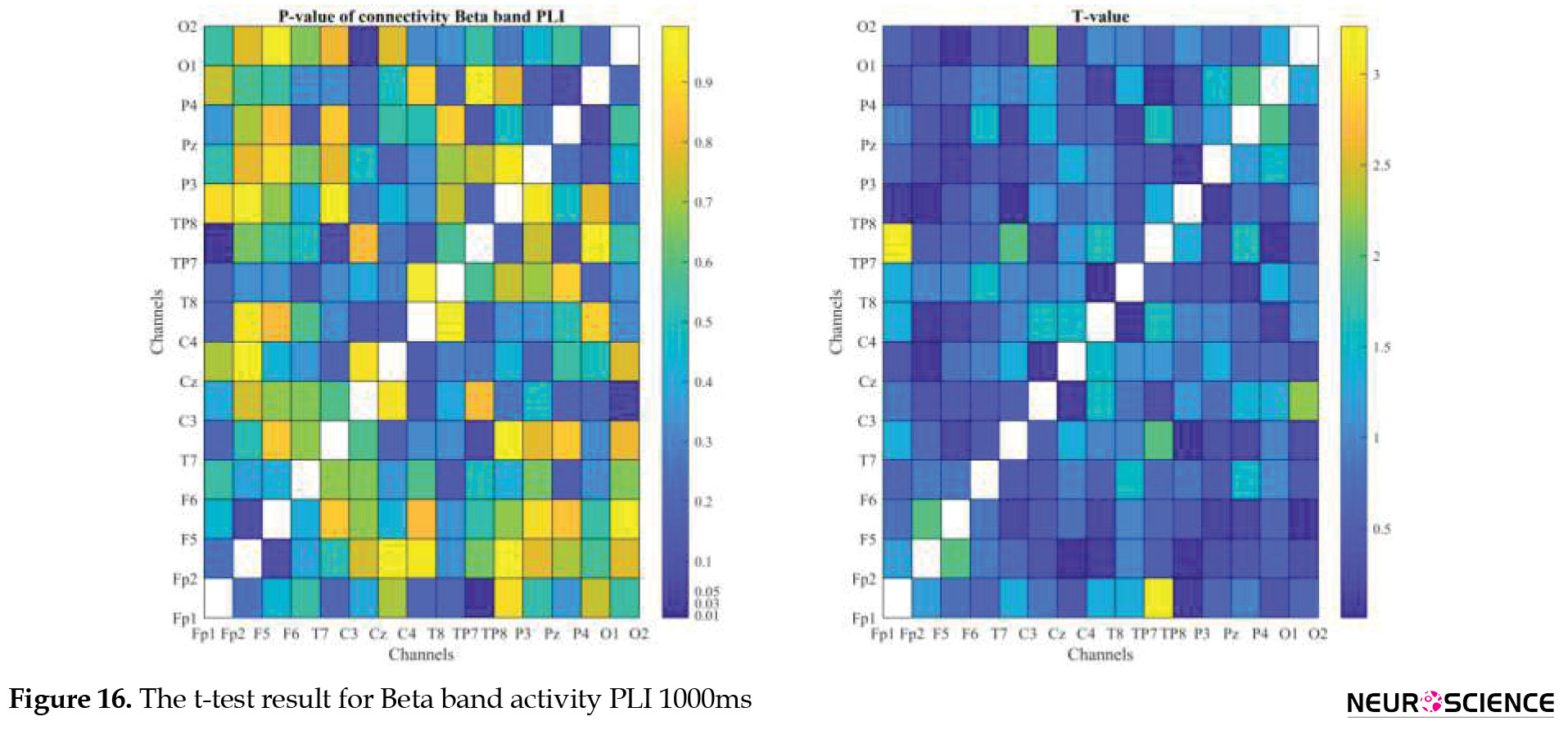



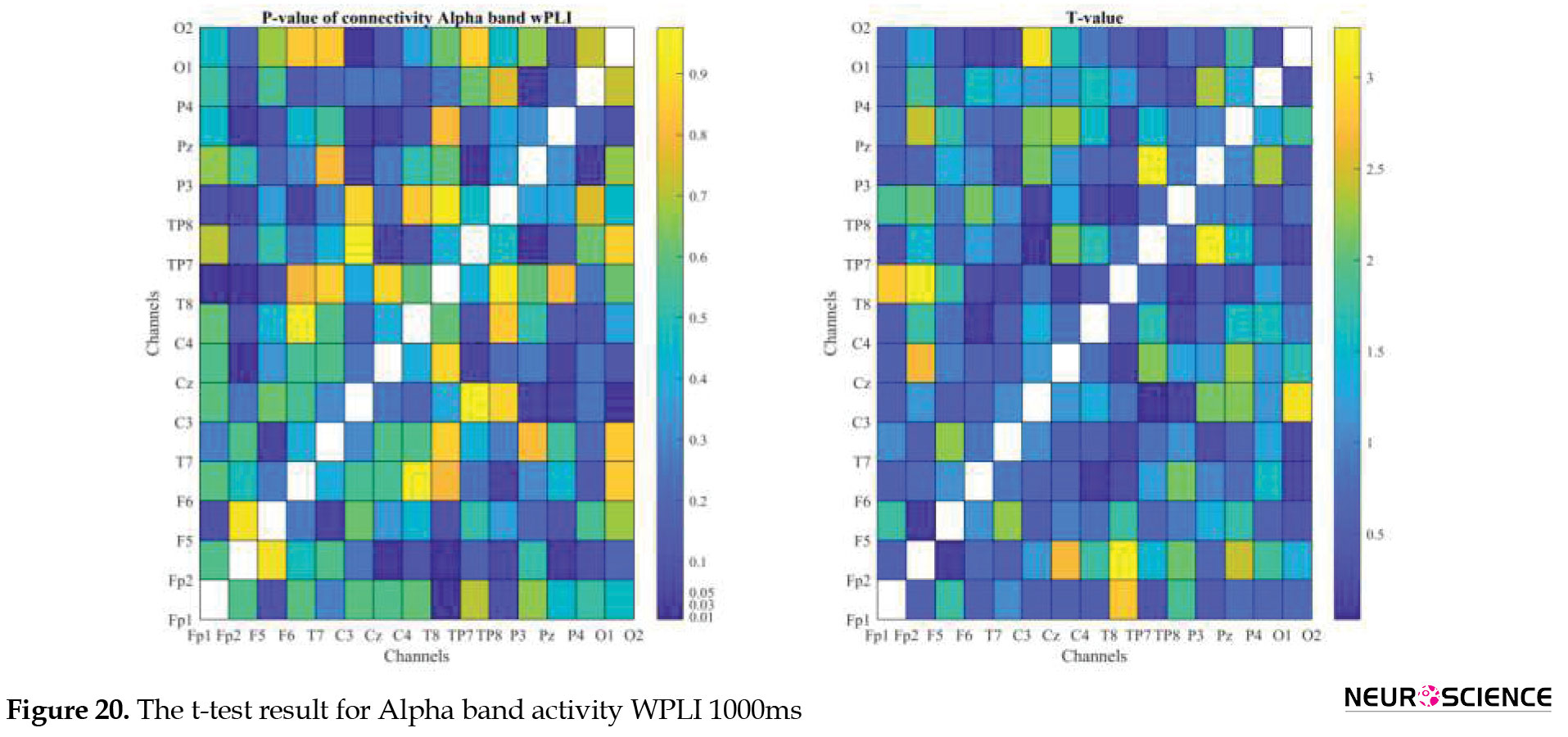
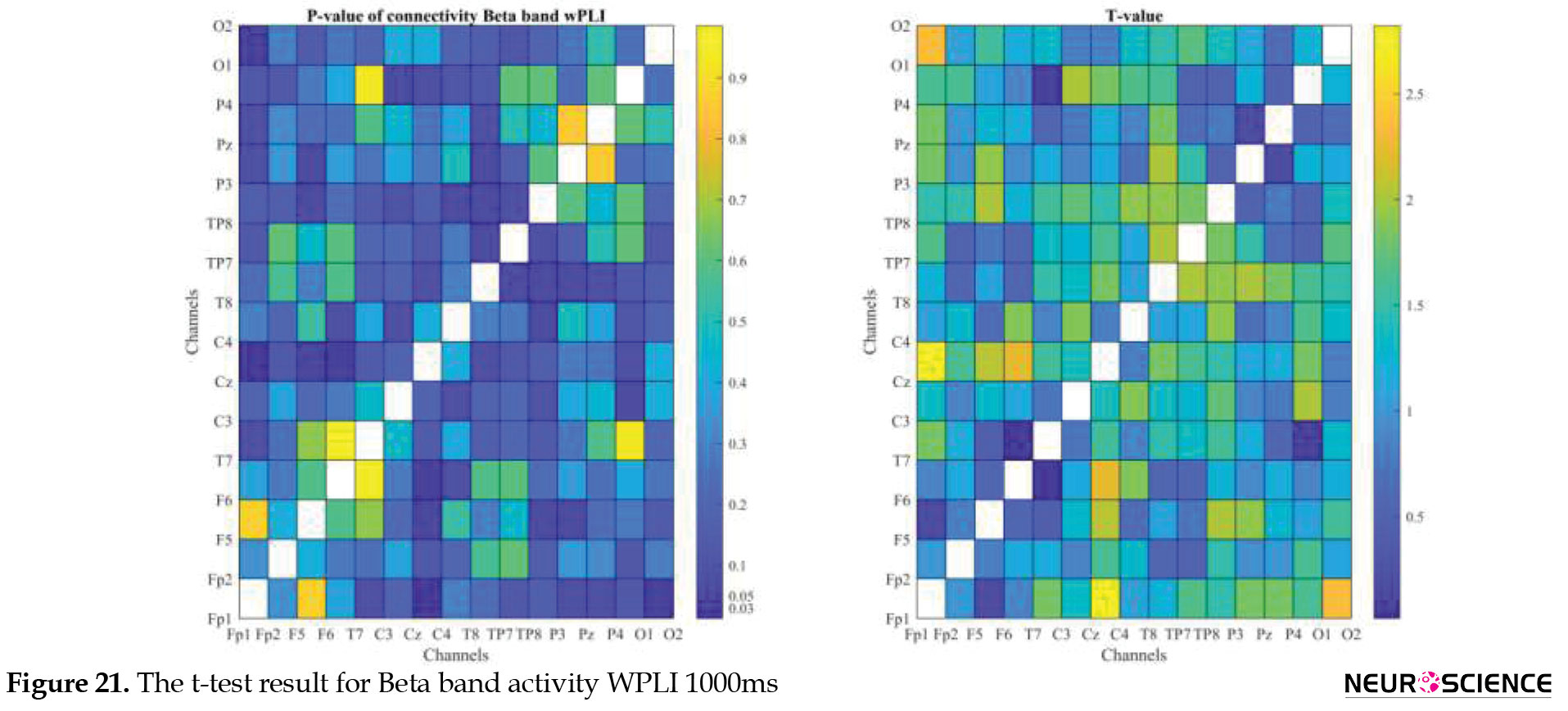

The visual representation of the results obtained from the calculation of PLA and WPLA was used to better display the functional connectivity of different brain parts. This process is performed using the t-test in two stages, one before and one after performing the experimental task. The report of the best values (most effective values) is presented in Tables 2, 3, 4, 5.
.jpg)
.jpg)
.jpg)
.jpg)
The effect of training between the first to the sixth day and the results of calculating the PLI and WPLI for different channels and in different frequency bands for the first and last day of the test, and also the results of the paired t-test for the group of participants only in some channels showed significant changes.
4. Discussion
The study limitations are the gender of participants, the long experimental process and its repetition in six consecutive days, and simultaneous task design for both short and long periods. As mentioned, the issue of time perception is a vital challenge that human beings face in various aspects of their lives. Researchers have constantly been challenged in how to calculate it and understand its mechanism for each individual. In the present study, based on the temporal perception and comparing the timing of auditory stimuli, we seek to show the functional relationships of neural network formation related to learning temporal perception. We aimed to understand how the hidden information of auditory stimuli (time intervals) is encoded in the content of brain signals. For this purpose, an intervention model with the help of acoustic stimuli with a distance of less than one second (short) and one second (long) was designed to study the learning process of temporal differentiation in healthy individuals. Experimental findings showed an overall improvement in the ability to store and compare learning stimuli in all target group members.
By considering the functional connections between the EEG channels, one can find the areas that have had the most involvement in the learning process. The areas involved in the time analysis process can be determined based on the sound system’s intrinsic characteristics and anatomical structures. For this purpose, we used the connection matrix based on PLI and WPLI at different time intervals and in standard frequency bands. According to the topography of brain signals in standard bands, the activity of different regions can be clearly seen with a structure distributed in the middle and occipital regions. The present study focuses on the relationship between neural mechanisms and behavioral outcomes and whether a behavioral relationship between two inherently independent groups can be shown. For this purpose, the correlation test between behavioral and cognitive outcomes was used.
The group results confirmed that participants’ accuracy in differentiating time intervals improves as the learning process progresses. In other words, the distance between the auditory stimuli decreases as the learning process decreases. The behavioral results obtained in this study showed that individuals could learn time discrimination effectively. They recorded a time difference of 26ms for the short interval and 42ms for the long interval. Based on these results, it can be said that the learning process directly affects the mechanism of time perception. The hypothesis of the plasticity effect of the brain on the temporal perception mechanism was also confirmed. In addition, by examining the correlation between participants’ level of attention (using the IVA test) and the results of the N-back test, which is an indicator for measuring working memory performance, we concluded that learning time difference differentiation does not generally affect working memory.
On the other hand, with the help of the study results of functional connections of brain regions using PLI and WPLI, we can identify the channels with the most activity in differentiating the time intervals and the areas involved in this mechanism. These results are similar to the findings of previous studies (Almanza-Sepúlveda, Hernández-González, Hevia- Orozco, Amezcua-Gutiérrez, & Guevara, 2018; Azari et al., 2020; Hsieh & Ranganath, 2014; Shalbaf et al., 2015).
Previous studies have also reported the importance of theta waves for learning time sequences (Crivelli-Decker, Hsieh, Clarke, & Ranganath, 2018) and beta fluctuations in estimating time (Ghaderi et al., 2018). The findings of this study show that learning time discrimination alters the functional relationships between the occipital and temporal regions that play an essential role in encoding time intervals (Paton & Buonomano, 2018). In addition, the role of the complementary motor area in interval differentiation has been observed with significant changes in the central area after learning the time intervals, which confirms the results (Mendoza, Méndez, Pérez, Prado, & Merchant, 2018).
As far as we know, this is the first study to demonstrate the neural relationship between learning to discriminate over time; the results logically follow previous findings and show how changes in brain functional communication are associated with learning to discriminate over time. The periodic learning mechanism may not directly affect other higher cognitive functions such as working memory (based on the N-back results).
5. Conclusion
The results showed that most of the established connections related to learning the different time intervals were formed in the temporal, occipital, and middle regions. These results confirm the previous findings of the researchers. In fact, with increasing accuracy in recognizing auditory intervals in feedback-based learning practice, more connections are made between the involved areas of the brain. The results show that this learning process is generalized to auditory stimuli with different frequencies. However, it does not directly affect working memory performance. In addition, learning intermittent discrimination occurs with changes in functional communication in brain areas. Significant changes in the connections formed between different brain parts were observed mainly in the temporal, central, and parietal regions in the theta, gamma, and sometimes beta frequency bands. These areas have played a role in the perception of time. The results showed a direct relationship between beta frequency, the number of formed connections, and the ability to detect auditory intervals. However, interval learning was not directly related to working memory function, and changes in brain activity were distributed over the brain. The available findings emphasize the existence of a distributed system for the perception of time intervals, but the hypothesis of the central mechanism of the time perception system was not confirmed.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Shahid Beheshti University (Code: IR.SBU.REC.1399.040).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to Reza Khosrowabadi for his great supervision.
References
Almanza-Sepúlveda, M. L., Hernández-González, M., Hevia-Orozco, J. C., Amezcua-Gutiérrez, C., & Guevara, M. A. (2018). Verbal and visuospatial working memory during pregnancy: EEG correlation between the prefrontal and parietal cortices. Neurobiology of Learning and Memory, 148(July 2017), 1-7. [DOI:10.1016/j.nlm.2017.12.003] [PMID]
Azari, L., Mioni, G., Rousseau, R., & Grondin, S. (2020). An analysis of the processing of intramodal and intermodal time intervals. Attention, Perception, & Psychophysics, 82(3), 1473-1487. [DOI:10.3758/s13414-019-01900-7] [PMID]
Brown, T. A., Chorpita, B. F., Korotitsch, W., & Barlow, D. H. (1997). Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behaviour Research and Therapy, 35(1), 79-89. [DOI:10.1016/S0005-7967(96)00068-X]
Bruning, J. L., & Kintz, B. L. (1987). Computational handbook of statistics, 3rd ed. Scott, Foresman & Co. [Link]
Bueti, D., & Walsh, V. (2009). The parietal cortex and the representation of time, space, number, and other magnitudes. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1525), 1831- 1840. [DOI:10.1098/rstb.2009.0028] [PMID] [PMCID]
Buzsáki, G., & Llinás, R. (2017). Space and time in the brain. Science, 358(6362), 482-485. [DOI:10.1126/science.aan8869] [PMID] [PMCID]
Chauvigné, L. A. S., Gitau, K. M., & Brown, S. (2014). The neural basis of audio motor entrainment: An ALE meta-analysis. Frontiers in Human Neuroscience, 8, 776. [DOI:10.3389/fnhum.2014.00776] [PMID] [PMCID]
Chen, Y. C., Shore, D. I., Lewis, T. L., & Maurer, D. (2016). The development of the perception of audiovisual simultaneity. Journal of Experimental Child Psychology, 146, 17-33. [DOI:10.1016/j.jecp.2016.01.010] [PMID]
Coull, J. T., & Droit-Volet, S. (2018). An explicit understanding of duration develops implicitly through action. Trends in Cognitive Sciences, 22(10), 923-937. [DOI:10.1016/j.tics.2018.07.011] [PMID]
Crivelli-Decker, J., Hsieh, L.-T., Clarke, A., & Ranganath, C. (2018). Theta oscillations promote temporal sequence learning. Neurobiology of Learning and Memory, 153, 92-103. [DOI:10.1016/j.nlm.2018.05.001] [PMID]
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics, including independent component analysis. Journal of Neuroscience Methods, 134(1), 9-21. [DOI:10.1016/j.jneumeth.2003.10.009] [PMID]
Drayton, L., & Furman, M. (2018). Thy mind, thy brain, and time. Trends in Cognitive Sciences, 22(10), 841- 843. [DOI:10.1016/j.tics.2018.08.007] [PMID]
Gámez, J., Mendoza, G., Prado, L., Betancourt, A., & Merchant, H. (2019). The amplitude in periodic neural state trajectories underlies the tempo of rhythmic tapping. PLoS Biology, 17(4), e3000054. [DOI:10.1371/journal.pbio.3000054] [PMID] [PMCID]
Ghaderi, A. H., Moradkhani, S., Haghighatfard, A., Akrami, F., Khayyer, Z., & Balci, F. (2018). Time estimation and beta segregation: An EEG study and graph theoretical approach. PLoS One, 13(4), e0195380. [DOI:10.1371/journal.pone.0195380] [PMID] [PMCID]
Gili, T., Ciullo, V., & Spalletta, G. (2018). Metastable states of multiscale brain networks are keys to cracking the timing problem. Frontiers in Computational Neuroscience, 12, 75. [DOI:10.3389/fncom.2018.00075] [PMID] [PMCID]
Gonzalez-Castillo, J., & Bandettini, P. A. (2018). Task-based dynamic functional connectivity: Recent findings and open questions. NeuroImage, 180, 526-533. [DOI:10.1016/j.neuroimage.2017.08.006] [PMID] [PMCID]
Grondin, S. (2010). Timing and time perception: A review of recent behavioral and neuroscience findings and theoretical directions. Attention, Perception, & Psychophysics, 72(3), 561-582. [DOI:10.3758/APP.72.3.561] [PMID]
Hayashi, M. J., van der Zwaag, W., Bueti, D., & Kanai, R. (2018). Representations of time in human frontoparietal cortex. Communications Biology, 1(1), 1-10. [DOI:10.1038/s42003-018-0243-z] [PMID] [PMCID]
Hsieh, L. T., & Ranganath, C. (2014). Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. NeuroImage, 85, 721-729. [DOI:10.1016/j.neuroimage.2013.08.003] [PMID] [PMCID]
Imperatori, L. S., Betta, M., Cecchetti, L., Canales-Johnson, A., Ricciardi, E., Siclari, F., et al. (2019). EEG functional connectivity metrics wPLI and wSMI account for distinct types of brain functional interactions. Scientific Reports, 9(1), 8894. [DOI:10.1038/s41598-019-45289-7] [PMID] [PMCID]
Kane, M. J., Conway, A. R. A., Miura, T. K., & Colflesh, G. J. H. (2007). Working memory, attention control, and the N-back task question construct validity. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33(3), 615. [DOI:10.1037/0278-7393.33.3.615] [PMID]
Karmarkar, U. R., & Buonomano, D. V. (2003). Temporal specificity of perceptual learning in an auditory discrimination task. Learning and Memory, 10(2), 141-147. [DOI:10.1101/lm.55503] [PMID] [PMCID]
Lad, S. S., Hurley, R. A., & Taber, K. H. (2020). Temporal processing: Neural correlates and clinical relevance. Journal of Neuropsychiatry and Clinical Neurosciences, 32(2), 104-108. [DOI:10.1176/appi.neuropsych.19120342] [PMID]
Martel, A.-C., & Apicella, P. (2021). Temporal processing in the striatum: Interplay between midbrain dopamine neurons and striatal cholinergic interneurons. European Journal of Neuroscience, 53(7), 2090-2099. [DOI:10.1111/ejn.14741] [PMID]
Matthews, W. J., & Meck, W. H. (2016). Temporal cognition: Connecting subjective time to perception, attention, and memory. Psychological bulletin, 142(8), 865-907. [DOI:10.1037/bul0000045] [PMID]
Meck, W. H. (2006). Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research, 1109(1), 93-107. [DOI:10.1016/j.brainres.2006.06.031] [PMID]
Mendoza, G., Méndez, J. C., Pérez, O., Prado, L., & Merchant, H. (2018). Neural basis for categorical boundaries in the primate pre-SMA during relative categorization of time intervals. Nature Communications, 9(1), 1-17. [DOI:10.1038/s41467-018-03482-8] [PMID] [PMCID]
Merchant, H., Pérez, O., Bartolo, R., Méndez, J. C., Mendoza, G., Gámez, J., et al. (2015). Sensorimotor neural dynamics during isochronous tapping in the medial premotor cortex of the macaque. European Journal of Neuroscience, 41(5), 586-602. [DOI:10.1111/ejn.12811] [PMID]
Mioni, G., Grondin, S., Bardi, L., & Stablum, F. (2020). Understanding time perception through non-invasive brain stimulation techniques: A review of studies. Behavioral Brain Research, 377, 112232. [DOI:10.1016/j.bbr.2019.112232] [PMID]
Montchal, M. E., Reagh, Z. M., & Yassa, M. A. (2019). The lateral entorhinal cortex supports precise temporal memories in humans. Nature Neuroscience, 22(2), 284-288. [DOI:10.1038/s41593-018-0303-1] [PMID] [PMCID]
Nani, A., Manuello, J., Liloia, D., Duca, S., Costa, T., & Cauda, F. (2019). The neural correlates of time: a meta-analysis of neuroimaging studies. Journal of Cognitive Neuroscience, 31(12), 1796-1826. [DOI:10.1162/jocn_a_01459] [PMID]
Niso, G., Bruña, R., Pereda, E., Gutiérrez, R., Bajo, R., Maestú, F., & Del-Pozo, F. (2013). HERMES: Towards an integrated toolbox to characterize functional and effective brain connectivity. Neuroinformatics, 11(4), 405-434. [DOI:10.1007/s12021-013-9186-1] [PMID]
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97-113. [DOI:10.1016/0028-3932(71)90067-4]
Paton, J. J., & Buonomano, D. V. (2018). The neural basis of timing: Distributed mechanisms for diverse functions. Neuron, 98(4), 687-705. [DOI:10.1016/j.neuron.2018.03.045] [PMID] [PMCID]
Penrose, L. S., & Raven, J. C. (1936). A new series of perceptual tests: Preliminary communication. British Journal of Medical Psychology, 16(2), 97-104. [DOI:10.1111/j.2044-8341.1936.tb00690.x]
Powers, A. R., Hillock-Dunn, A., & Wallace, M. T. (2016). Generalization of multisensory perceptual learning. Scientific Reports, 6(September 2015), 1-9. [DOI:10.1038/srep23374] [PMID] [PMCID]
Protopapa, F., Hayashi, M. J., Kulashekhar, S., van der Zwaag, W., Battistella, G., Murray, M. M., … Bueti, D. (2019). Chronotopic maps in the human supplementary motor area. PLoS Biology, 17(3), e3000026. [DOI:10.1371/journal.pbio.3000026] [PMID] [PMCID]
Rouzbahani, M., Motie, A. (2017). Effective connectivity estimation based on emotion EEG signal by granger causality and directed transfer function. Journal of Advances in Cognitive Sciences, 19(2), 1-18. [Link]
Shalbaf, R., Behnam, H., Sleigh, J. W., Steyn-Ross, D. A., & Steyn-Ross, M. L. (2015). Frontal-temporal synchronization of EEG signals quantified by order patterns crosses recurrence analysis during propofol anesthesia. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 23(3), 468-474. [DOI:10.1109/TNSRE.2014.2350537] [PMID]
Siman-Tov, T., Granot, R. Y., Shany, O., Singer, N., Hendler, T., & Gordon, C. R. (2019). Is there a prediction network? Meta-analytic evidence for a cortical-subcortical network likely subserving prediction. Neuroscience & Biobehavioral Reviews, 105, 262-275. [DOI:10.1016/j.neubiorev.2019.08.012] [PMID]
Soares, S., Atallah, B. V, & Paton, J. J. (2016). Midbrain dopamine neurons control judgment of time. Science, 354(6317), 1273-1277. [DOI:10.1126/science.aah5234] [PMID]
Stam, C. J., Nolte, G., & Daffertshofer, A. (2007). Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Human Brain Mapping, 28(11), 1178-1193. [DOI:10.1002/hbm.20346] [PMID] [PMCID]
Sugar, J., & Moser, M.-B. (2019). Episodic memory: Neuronal codes for what, where, and when. Hippocampus, 29(12), 1190-1205. [DOI:10.1002/hipo.23132] [PMID]
Tamburro, G., di Fronso, S., Robazza, C., Bertollo, M., & Comani, S. (2020). Modulation of brain functional connectivity and efficiency during an endurance cycling task: A source-level EEG and graph theory approach. Frontiers in Human Neuroscience, 14, 243. [DOI:10.3389/fnhum.2020.00243] [PMID] [PMCID]
Tinius, T. P. (2003). The integrated visual and auditory continuous performance test as a neuropsychological measure. Archives of Clinical Neuropsychology, 18(5), 439-454. [DOI:10.1016/S0887-6177(02)00144-0] [PMID]
Umbach, G., Kantak, P., Jacobs, J., Kahana, M., Pfeiffer, B. E., Sperling, M., et al. (2020). Time cells in the human Hippocampus and entorhinal cortex support episodic memory. Proceedings of the National Academy of Sciences, 117(45), 28463-28474. [DOI:10.1073/pnas.2013250117] [PMID] [PMCID]
Villalonga, M. B., Sussman, R. F., & Sekuler, R. (2021). Perceptual timing precision with vibrotactile, auditory, and multisensory stimuli. Attention, Perception, & Psychophysics, 83(5), 2267-2280. [DOI:10.3758/s13414-021-02254-9] [PMID]
Vinck, M., Oostenveld, R., Van Wingerden, M., Battaglia, F., & Pennartz, C. M. A. (2011). An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise, and sample-size bias. Neuroimage, 55(4), 1548-1565. [DOI:10.1016/j.neuroimage.2011.01.055] [PMID]
Wiener, M., Turkeltaub, P., & Coslett, H. B. (2010). The image of time: A voxel-wise meta-analysis. NeuroImage, 49(2), 1728-1740. [DOI:10.1016/j.neuroimage.2009.09.064] [PMID]
Wittmann, M., Simmons, A. N., Aron, J. L., & Paulus, M. P. (2010). Accumulation of neural activity in the posterior insula encodes the passage of time. Neuropsychologia, 48(10), 3110-3120. [DOI:10.1016/j.neuropsychologia.2010.06.023] [PMID] [PMCID]
Wright, B. A., Buonomano, D. V., Mahncke, H. W., & Merzenich, M. M. (1997). Learning and generalization of auditory temporal-interval discrimination in humans. Journal of Neuroscience, 17(10), 3956-3963. [DOI:10.1523/jneurosci.17-10-03956.1997] [PMID] [PMCID]
Wu, S., Shankar Gupta, D., Banerjee, A., Roy, D., & Piras, F. (2020). Editorial: Temporal structure of neural processes coupling sensory, motor and cognitive functions of the brain. Frontiers in Computational Neuroscience, 14, 73. [DOI:10.3389/fncom.2020.00073] [PMID] [PMCID]
Zippo, A. G., Della Rosa, P. A., Castiglioni, I., & Biella, G. E. M. (2018). Alternating dynamics of segregation and integration in human EEG functional networks during working-memory task. Neuroscience, 371, 191-206. [DOI:10.1016/j.neuroscience.2017.12.004] [PMID]
According to various documents, physical senses are processed in specific areas of the brain, but no specific area of the brain processes the sense of internal time (Azari, Mioni, Rousseau, & Grondin, 2020; Matthews & Meck, 1991; Meck, 2006; Mioni, Grondin, Bardi, & Stablum, 2020; Nani et al., 2019; Tamburro, di Fronso, Robazza, Bertollo, & Comani, 2020; Umbach et al., 2020; Wiener, Turkeltaub, & Coslett, 2010; Wu, Shankar Gupta, Banerjee, Roy, & Piras, 2020). Perception of time, which is one of the most vital functions of the brain (internal experience of time), is an abstract concept that is created based on the information recorded in the brain (internal and external senses in addition to memory) with the help of a network of its regions. Of course, each region may refer to a different aspect of time (Buzsáki & Llinás, 2017; Coull & Droit-Volet, 2018; Gili, Ciullo, & Spalletta, 2018; Matthews & Meck, 2016; Nani et al., 2019).
In related research that has been done so far, potential mechanisms related to a diverse set of brain regions have been discovered, some of which are as follows: pre-supplementary motor regions (Gámez, Mendoza, Prado, Betancourt, & Merchant, 2019; Merchant et al., 2015; Protopapa et al., 2019), parietal regions (Bueti & Walsh, 2009; Hayashi, van der Zwaag, Bueti, & Kanai, 2018), insula (Wittmann, Simmons, Aron, & Paulus, 2010), midbrain dopaminergic neurons (Martel & Apicella, 2021; Soares, Atallah, & Paton, 2016), hippocampus and entorhinal cortex (Montchal, Reagh, & Yassa, 2019; Sugar & Moser, 2019; Umbach et al., 2020).
Therefore, the study and discovery of networks related to temporal perception are among the fascinating topics in the field of brain behavior that researchers have always attended. On the other hand, it is observed in many human experiences (such as learning music) that a person can better understand the accuracy of temporal events through practice (Chauvigné, Gitau, & Brown, 2014; Drayton & Furman, 2018; Siman-Tov et al., 2019). This diversity of neural areas may be related to human perception of time. Time perception reflects the nervous system’s dynamics because these dynamics result from the objective clock (Lad, Hurley, & Taber, 2020).
One of the primary factors in learning is the creation of new neural connections in the brain; these connections are created when the brain is engaged in a learning process. Many experimental results show that active interaction is the background of changes in the brain. That is, learning will not occur unless the brain is involved in an active engagement; for example, listening to a lecture or giving a presentation will never lead to permanent learning. The things that enhance training include facilitation, simulation, play, and role-playing that stimulate active interaction.
One of the criteria that show the effect of changes in the brain regions well is the functional connectivity (FC) criteria which shows the significant changes in the connection of different parts of the brain during the learning process (Shalbaf, Behnam, Sleigh, Steyn-Ross, & Steyn-Ross, 2015; Zippo, Della Rosa, Castiglioni, & Biella, 2018). On the other hand, electroencephalography (EEG) is a helpful tool for analyzing how to understand cognitive time intervals (inms). The combination of these two tools, namely functional connectivity, and EEG, can provide valuable information on how to record and form connections of parts related to the learning process of differentiation of time intervals (Gonzalez-Castillo & Bandettini, 2018). However, EEG acquisition suffers from the problem of volume conduction caused by the propagation of electric fields generated by a mainstream source to all (or more) of the surrounding sensors located on the scalp (Rouzbahani & Motie, 2017; Gonzalez-Castillo & Bandettini, 2018). This problem is a linear behavior. In such a case, standard FC estimation methods, such as coherence or cross-information, lead to the identification of functional connections that do not reflect actual interregional interactions in the brain (Imperatori et al., 2019).
Alternative methods can determine the functional connections and solve this problem. These methods include weighted phase lag index (WPLI) and phase lag index (PLI), which have been widely used in many studies to estimate the functional connectivity of EEG. Based on the obtained results, these two methods identify relative changes in the functional connectivity of the brain in conditions associated with changes in the level of consciousness (Gonzalez- Castillo & Bandettini, 2018).
The phase lag index was introduced by Stam, Nolte, & Daffertshofer (2007). Its main idea is not to pay attention to the phase lock, which is about the 0-axis phase difference. This criterion eliminates the effects of volume transfer (there is a risk of ignoring the actual immediate interactions). PLI can be the phase difference ratio between signals in multiple experiments above or below the zero-degree line (i.e., the true axis WPLI calculates phase synchronization, which is mainly used for linear interactions but also seems suitable for nonlinear coupling. The values of both indicators are in the range of 0 to one. Unlike PLI, WPLI weights the cross-spectrum according to the magnitude of the imaginary component. This issue allows it to limit the influence of cross-spectrum elements around the real axes, which risk changing their “true” sign with small noise perturbations. Such an index of phase synchronization was proposed by Vinck et al. This index is based only on the imaginary component of the cross-spectrum, which implies robustness to noise because uncorrelated noise sources will cause an increase in signal power. It has been shown that WPLI outperforms PLI, coherence, and imaginary coherence (IC) with real, local field potentials (LFP) data (Vinck et al., 2011).
As mentioned, the issue of time perception is a vital challenge that human beings face in various aspects of their lives. Researchers have constantly been challenged in calculating it and understanding its mechanism for each individual (Chen, Shore, Lewis, & Maurer, 2016; Grondin, 2010; Powers, Hillock-Dunn, & Wallace, 2016; Villalonga, Sussman, & Sekuler, 2021). In the present study, which is based on temporal perception, by comparing the timing of auditory stimuli (Azari et al., 2020; Ghaderi et al., 2018), we seek to show the functional relationships of neural network formation related to learning temporal perception. We aimed to understand how the hidden information of auditory stimuli (time intervals) is encoded in the content of brain signals. For this purpose, an intervention model with the help of acoustic stimuli with a distance of less than one second (short) and one second (long) was designed to study the learning process of temporal differentiation in healthy individuals. Experimental findings showed an overall improvement in the ability to store and compare learning stimuli in all target group members.
2. Materials and Methods
Ethical consideration
Ethical considerations of our study method have been approved by the Ethics Review Committee of Shahid Beheshti University. It is noteworthy that all stages of study and development methods have been approved and written by the Ethics Committee at the relevant university with the reference code I.R.SBU.REC.1399.040. The complete scenario of this research was explained to each participant, who voluntarily signed the relevant form and expressed their consent to participate in this test. These individuals had no previous information about assigning discrimination time intervals and have not been paid.
Study participants
Participants in this study were selected from undergraduate students in Medical Engineering, Computer Engineering, and Nursing at the Dezful Branch, Islamic Azad University, Iran. The total number of participants is 16 healthy right-handed volunteers, including 11 men and 5 women aged 22±1.7 years. Participants were tested before the preliminary test to ensure their ability to participate in the test. For this purpose, IQ (Penrose & Raven, 1936), depression, anxiety, and stress test (DASS42) (Brown, Chorpita, Korotitsch, & Barlow, 1997) and Edinburgh handedness test (Oldfield, 1971) were taken.
To assess the physical and mental condition of the individuals, they were asked to provide medical records, according to which participants had no hearing, memory, neurological impairment, pain status, or medication use that affected EEG data collection.
Before data collection, all the participants were asked not to consume alcohol or other beverages that contain caffeine which could affect the EEG data for 48h. However, two participants were excluded from the group due to their inability to learn the test. Also, one of the participants withdrew from the test for personal reasons.
Study materials
Participants cooperated in three tests: behavioral, cognitive, and neurophysiological. According to the scenario shown in Figure 1, the participants had to take one day for a preliminary test, including the Raven IQ test, the Edinburgh hand superiority test, and the DASS42. This work aimed to determine the initial status of the parameters that were thought to affect the participants’ learning process. The integrated visual and auditory (IVA) (Tinius, 2003) and N-back (Kane, Conway, Miura, & Colflesh, 2007) tests were also used to assess attention and working memory performance. Then, they conducted daily training sessions for 5 consecutive working days (except weekends). The experiments were performed in a room with controlled light and temperature without electrical interference. People were taught to focus only on auditory stimuli when sitting comfortably and without extra movement. In addition, they were asked not to think about anything else.
In the behavioral test, two time intervals were used simultaneously to prevent the participants’ mental adaptation to the test process to be a tool to test their perception from a neurological point of view. Participants were asked to identify standard and comparative intervals while focusing on the frequency of the sound stimulus. A modified scenario was used to determine the mechanism associated with learning time discrimination (Karmarkar & Buonomano, 2003). This stimulation protocol was used for all daily experiments, which general structure can be seen in Figure 1.
.jpg)
Study methods
In the first round of the work, EEG data (sampling frequency 256Hz) were recorded from the subject for 2 min with eyes closed and 2 min with eyes open without any stimulation. In this test, the electrode impedance was less than 10kΩ. Immediately after this stage, with the announcement of a short beep (1ms), the first round of trials was played for the participant, and the EEG signal was collected simultaneously. The trials were played similarly at the end of the first round and after a one-minute break in the second block. In addition, the same discipline was performed at the end of the third round after a one-minute break.
In each test round for the subject, stimuli consisting of two sounds with specific frequencies (4kHz and 1kHz) were played. Each pair of time intervals (standard and comparative) related to sound stimuli were separated by a fixed time interval (T) (Figure 1a and 1b). To avoid subjective adaptation, the fixed time intervals (T) of these pairs of intervals were randomly selected from the set (500, 600, or 700ms) for the short distance and (700,800 or 900ms) for the long distance.
Participants on the first day of the study could listen to the aforementioned audio structure in any number. Each trial consisted of playing test stimuli consisting of a pair of sounds shorter or longer than a pair of sounds with a standard interval (target stimulus) that generally had a duration equal to (T±Δt) (Figure 1). People were instructed to determine if the first or second interval was shorter or longer than the standard interval after hearing the sound stimuli through headphones by pressing one of the two keys on the test table. The tones were 15ms with a 5 on/off slope. The experiments were set up in such a way that on the first day, people were trained in cognitive, behavioral, and neuromarkers tests, and in the next five working days, in parallel with the learning process, the neuro markers, and cognitive tests were recorded
This training process was performed in four blocks of 40 trials with experimental stimuli adjusted for a long time randomly and intricately in each block. The method was used to determine the threshold values (Wright, Buonomano, Mahncke, & Merzenich, 1997). During each test block, an adaptive function reduces the comparison interval by as much as Δt after three correct answers but increases by the same amount of Δt for each incorrect answer. The Δt values were recorded at pivot points (decrease to increase or vice versa). Since the stimuli in the protocols had spaces equal to T±δt, the threshold is twice the mean of the total inverse values except for the first three trials. The effective learning curve is determined by linear orthogonal trend analysis (Bruning & Kintz, 1987).
Data acquisition and analysis
In this study, 16 electrodes were used to obtain EEG signals, which were arranged according to the international standard 10-10 (FP1, FP2, F5, F6, T7, C3, CZ, C4, T8, TP7, TP8, P3, P.Z., P4, O1, and O2). The sampling rate was set at 256 samples per second. Brain signals were recorded on the first and last day of the experiments to determine learning effects. With the help of PLI and WPLI, neural networks related to functional connectivity of different brain parts involved in learning time perception were investigated. In other words, brain areas associated with this process were examined to demonstrate the effect of time learning discrimination on neural activity.
Cognitive indicators included IQ, stress, depression, and anxiety. In addition, to assess a person’s level of auditory attention before the behavioral test, the IVA test (focusing on speed and alertness) was performed at the beginning of all training sessions. Standard and comparative time difference parameters were used to analyze the behavioral data of the test sessions. After the behavioral test, the N-back test (levels one and three) was performed to investigate the effect of temporal perception learning on working memory performance.
To prepare the obtained EEG signal, preprocessing operations were performed using EEGLAB toolbox version 14.1.2b (Delorme & Makeig, 2004) in MATLAB 2016b software, including bandpass filtering 1-40 Hz, trials segmentation, removing eye movement/blinking, and muscular artifact using independent component analysis approach and removing still noisy segments.
Then, to separate EEG data in ordinary frequency bands such as delta [1-4 Hz], theta [4-8 Hz], alpha [8-13 Hz], beta [30-30 Hz], gamma [30-40 Hz] as well as the calculation and analysis of the PLI and WPLI, the HERMES Toolbox (Niso et al., 2013) was utilized. Since EEG data were recorded only on the first and last day of the experiment, the effect of time interval discrimination learning on the EEG was assessed using the paired t-test.
3. Results
The results of cognitive and behavioral tests are reported in Table 1.
.jpg)
The Δt index depends on the short- and long-time intervals, which shows behavioral changes for the under-training group. Figure 2 shows the group learning chart for short and long periods.
.jpg)
PLI and WPLI were used to determine the status of functional connections. A statistical test was used to determine the changes in functional connections between the first and sixth days of the test. Data based on each channel and the delta, theta, alpha, beta, and gamma bands of brain signals were examined separately. The results of the tests performed can be seen in Figures 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22.
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)











The visual representation of the results obtained from the calculation of PLA and WPLA was used to better display the functional connectivity of different brain parts. This process is performed using the t-test in two stages, one before and one after performing the experimental task. The report of the best values (most effective values) is presented in Tables 2, 3, 4, 5.
.jpg)
.jpg)
.jpg)
.jpg)
The effect of training between the first to the sixth day and the results of calculating the PLI and WPLI for different channels and in different frequency bands for the first and last day of the test, and also the results of the paired t-test for the group of participants only in some channels showed significant changes.
4. Discussion
The study limitations are the gender of participants, the long experimental process and its repetition in six consecutive days, and simultaneous task design for both short and long periods. As mentioned, the issue of time perception is a vital challenge that human beings face in various aspects of their lives. Researchers have constantly been challenged in how to calculate it and understand its mechanism for each individual. In the present study, based on the temporal perception and comparing the timing of auditory stimuli, we seek to show the functional relationships of neural network formation related to learning temporal perception. We aimed to understand how the hidden information of auditory stimuli (time intervals) is encoded in the content of brain signals. For this purpose, an intervention model with the help of acoustic stimuli with a distance of less than one second (short) and one second (long) was designed to study the learning process of temporal differentiation in healthy individuals. Experimental findings showed an overall improvement in the ability to store and compare learning stimuli in all target group members.
By considering the functional connections between the EEG channels, one can find the areas that have had the most involvement in the learning process. The areas involved in the time analysis process can be determined based on the sound system’s intrinsic characteristics and anatomical structures. For this purpose, we used the connection matrix based on PLI and WPLI at different time intervals and in standard frequency bands. According to the topography of brain signals in standard bands, the activity of different regions can be clearly seen with a structure distributed in the middle and occipital regions. The present study focuses on the relationship between neural mechanisms and behavioral outcomes and whether a behavioral relationship between two inherently independent groups can be shown. For this purpose, the correlation test between behavioral and cognitive outcomes was used.
The group results confirmed that participants’ accuracy in differentiating time intervals improves as the learning process progresses. In other words, the distance between the auditory stimuli decreases as the learning process decreases. The behavioral results obtained in this study showed that individuals could learn time discrimination effectively. They recorded a time difference of 26ms for the short interval and 42ms for the long interval. Based on these results, it can be said that the learning process directly affects the mechanism of time perception. The hypothesis of the plasticity effect of the brain on the temporal perception mechanism was also confirmed. In addition, by examining the correlation between participants’ level of attention (using the IVA test) and the results of the N-back test, which is an indicator for measuring working memory performance, we concluded that learning time difference differentiation does not generally affect working memory.
On the other hand, with the help of the study results of functional connections of brain regions using PLI and WPLI, we can identify the channels with the most activity in differentiating the time intervals and the areas involved in this mechanism. These results are similar to the findings of previous studies (Almanza-Sepúlveda, Hernández-González, Hevia- Orozco, Amezcua-Gutiérrez, & Guevara, 2018; Azari et al., 2020; Hsieh & Ranganath, 2014; Shalbaf et al., 2015).
Previous studies have also reported the importance of theta waves for learning time sequences (Crivelli-Decker, Hsieh, Clarke, & Ranganath, 2018) and beta fluctuations in estimating time (Ghaderi et al., 2018). The findings of this study show that learning time discrimination alters the functional relationships between the occipital and temporal regions that play an essential role in encoding time intervals (Paton & Buonomano, 2018). In addition, the role of the complementary motor area in interval differentiation has been observed with significant changes in the central area after learning the time intervals, which confirms the results (Mendoza, Méndez, Pérez, Prado, & Merchant, 2018).
As far as we know, this is the first study to demonstrate the neural relationship between learning to discriminate over time; the results logically follow previous findings and show how changes in brain functional communication are associated with learning to discriminate over time. The periodic learning mechanism may not directly affect other higher cognitive functions such as working memory (based on the N-back results).
5. Conclusion
The results showed that most of the established connections related to learning the different time intervals were formed in the temporal, occipital, and middle regions. These results confirm the previous findings of the researchers. In fact, with increasing accuracy in recognizing auditory intervals in feedback-based learning practice, more connections are made between the involved areas of the brain. The results show that this learning process is generalized to auditory stimuli with different frequencies. However, it does not directly affect working memory performance. In addition, learning intermittent discrimination occurs with changes in functional communication in brain areas. Significant changes in the connections formed between different brain parts were observed mainly in the temporal, central, and parietal regions in the theta, gamma, and sometimes beta frequency bands. These areas have played a role in the perception of time. The results showed a direct relationship between beta frequency, the number of formed connections, and the ability to detect auditory intervals. However, interval learning was not directly related to working memory function, and changes in brain activity were distributed over the brain. The available findings emphasize the existence of a distributed system for the perception of time intervals, but the hypothesis of the central mechanism of the time perception system was not confirmed.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Shahid Beheshti University (Code: IR.SBU.REC.1399.040).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to Reza Khosrowabadi for his great supervision.
References
Almanza-Sepúlveda, M. L., Hernández-González, M., Hevia-Orozco, J. C., Amezcua-Gutiérrez, C., & Guevara, M. A. (2018). Verbal and visuospatial working memory during pregnancy: EEG correlation between the prefrontal and parietal cortices. Neurobiology of Learning and Memory, 148(July 2017), 1-7. [DOI:10.1016/j.nlm.2017.12.003] [PMID]
Azari, L., Mioni, G., Rousseau, R., & Grondin, S. (2020). An analysis of the processing of intramodal and intermodal time intervals. Attention, Perception, & Psychophysics, 82(3), 1473-1487. [DOI:10.3758/s13414-019-01900-7] [PMID]
Brown, T. A., Chorpita, B. F., Korotitsch, W., & Barlow, D. H. (1997). Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behaviour Research and Therapy, 35(1), 79-89. [DOI:10.1016/S0005-7967(96)00068-X]
Bruning, J. L., & Kintz, B. L. (1987). Computational handbook of statistics, 3rd ed. Scott, Foresman & Co. [Link]
Bueti, D., & Walsh, V. (2009). The parietal cortex and the representation of time, space, number, and other magnitudes. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1525), 1831- 1840. [DOI:10.1098/rstb.2009.0028] [PMID] [PMCID]
Buzsáki, G., & Llinás, R. (2017). Space and time in the brain. Science, 358(6362), 482-485. [DOI:10.1126/science.aan8869] [PMID] [PMCID]
Chauvigné, L. A. S., Gitau, K. M., & Brown, S. (2014). The neural basis of audio motor entrainment: An ALE meta-analysis. Frontiers in Human Neuroscience, 8, 776. [DOI:10.3389/fnhum.2014.00776] [PMID] [PMCID]
Chen, Y. C., Shore, D. I., Lewis, T. L., & Maurer, D. (2016). The development of the perception of audiovisual simultaneity. Journal of Experimental Child Psychology, 146, 17-33. [DOI:10.1016/j.jecp.2016.01.010] [PMID]
Coull, J. T., & Droit-Volet, S. (2018). An explicit understanding of duration develops implicitly through action. Trends in Cognitive Sciences, 22(10), 923-937. [DOI:10.1016/j.tics.2018.07.011] [PMID]
Crivelli-Decker, J., Hsieh, L.-T., Clarke, A., & Ranganath, C. (2018). Theta oscillations promote temporal sequence learning. Neurobiology of Learning and Memory, 153, 92-103. [DOI:10.1016/j.nlm.2018.05.001] [PMID]
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics, including independent component analysis. Journal of Neuroscience Methods, 134(1), 9-21. [DOI:10.1016/j.jneumeth.2003.10.009] [PMID]
Drayton, L., & Furman, M. (2018). Thy mind, thy brain, and time. Trends in Cognitive Sciences, 22(10), 841- 843. [DOI:10.1016/j.tics.2018.08.007] [PMID]
Gámez, J., Mendoza, G., Prado, L., Betancourt, A., & Merchant, H. (2019). The amplitude in periodic neural state trajectories underlies the tempo of rhythmic tapping. PLoS Biology, 17(4), e3000054. [DOI:10.1371/journal.pbio.3000054] [PMID] [PMCID]
Ghaderi, A. H., Moradkhani, S., Haghighatfard, A., Akrami, F., Khayyer, Z., & Balci, F. (2018). Time estimation and beta segregation: An EEG study and graph theoretical approach. PLoS One, 13(4), e0195380. [DOI:10.1371/journal.pone.0195380] [PMID] [PMCID]
Gili, T., Ciullo, V., & Spalletta, G. (2018). Metastable states of multiscale brain networks are keys to cracking the timing problem. Frontiers in Computational Neuroscience, 12, 75. [DOI:10.3389/fncom.2018.00075] [PMID] [PMCID]
Gonzalez-Castillo, J., & Bandettini, P. A. (2018). Task-based dynamic functional connectivity: Recent findings and open questions. NeuroImage, 180, 526-533. [DOI:10.1016/j.neuroimage.2017.08.006] [PMID] [PMCID]
Grondin, S. (2010). Timing and time perception: A review of recent behavioral and neuroscience findings and theoretical directions. Attention, Perception, & Psychophysics, 72(3), 561-582. [DOI:10.3758/APP.72.3.561] [PMID]
Hayashi, M. J., van der Zwaag, W., Bueti, D., & Kanai, R. (2018). Representations of time in human frontoparietal cortex. Communications Biology, 1(1), 1-10. [DOI:10.1038/s42003-018-0243-z] [PMID] [PMCID]
Hsieh, L. T., & Ranganath, C. (2014). Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. NeuroImage, 85, 721-729. [DOI:10.1016/j.neuroimage.2013.08.003] [PMID] [PMCID]
Imperatori, L. S., Betta, M., Cecchetti, L., Canales-Johnson, A., Ricciardi, E., Siclari, F., et al. (2019). EEG functional connectivity metrics wPLI and wSMI account for distinct types of brain functional interactions. Scientific Reports, 9(1), 8894. [DOI:10.1038/s41598-019-45289-7] [PMID] [PMCID]
Kane, M. J., Conway, A. R. A., Miura, T. K., & Colflesh, G. J. H. (2007). Working memory, attention control, and the N-back task question construct validity. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33(3), 615. [DOI:10.1037/0278-7393.33.3.615] [PMID]
Karmarkar, U. R., & Buonomano, D. V. (2003). Temporal specificity of perceptual learning in an auditory discrimination task. Learning and Memory, 10(2), 141-147. [DOI:10.1101/lm.55503] [PMID] [PMCID]
Lad, S. S., Hurley, R. A., & Taber, K. H. (2020). Temporal processing: Neural correlates and clinical relevance. Journal of Neuropsychiatry and Clinical Neurosciences, 32(2), 104-108. [DOI:10.1176/appi.neuropsych.19120342] [PMID]
Martel, A.-C., & Apicella, P. (2021). Temporal processing in the striatum: Interplay between midbrain dopamine neurons and striatal cholinergic interneurons. European Journal of Neuroscience, 53(7), 2090-2099. [DOI:10.1111/ejn.14741] [PMID]
Matthews, W. J., & Meck, W. H. (2016). Temporal cognition: Connecting subjective time to perception, attention, and memory. Psychological bulletin, 142(8), 865-907. [DOI:10.1037/bul0000045] [PMID]
Meck, W. H. (2006). Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research, 1109(1), 93-107. [DOI:10.1016/j.brainres.2006.06.031] [PMID]
Mendoza, G., Méndez, J. C., Pérez, O., Prado, L., & Merchant, H. (2018). Neural basis for categorical boundaries in the primate pre-SMA during relative categorization of time intervals. Nature Communications, 9(1), 1-17. [DOI:10.1038/s41467-018-03482-8] [PMID] [PMCID]
Merchant, H., Pérez, O., Bartolo, R., Méndez, J. C., Mendoza, G., Gámez, J., et al. (2015). Sensorimotor neural dynamics during isochronous tapping in the medial premotor cortex of the macaque. European Journal of Neuroscience, 41(5), 586-602. [DOI:10.1111/ejn.12811] [PMID]
Mioni, G., Grondin, S., Bardi, L., & Stablum, F. (2020). Understanding time perception through non-invasive brain stimulation techniques: A review of studies. Behavioral Brain Research, 377, 112232. [DOI:10.1016/j.bbr.2019.112232] [PMID]
Montchal, M. E., Reagh, Z. M., & Yassa, M. A. (2019). The lateral entorhinal cortex supports precise temporal memories in humans. Nature Neuroscience, 22(2), 284-288. [DOI:10.1038/s41593-018-0303-1] [PMID] [PMCID]
Nani, A., Manuello, J., Liloia, D., Duca, S., Costa, T., & Cauda, F. (2019). The neural correlates of time: a meta-analysis of neuroimaging studies. Journal of Cognitive Neuroscience, 31(12), 1796-1826. [DOI:10.1162/jocn_a_01459] [PMID]
Niso, G., Bruña, R., Pereda, E., Gutiérrez, R., Bajo, R., Maestú, F., & Del-Pozo, F. (2013). HERMES: Towards an integrated toolbox to characterize functional and effective brain connectivity. Neuroinformatics, 11(4), 405-434. [DOI:10.1007/s12021-013-9186-1] [PMID]
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97-113. [DOI:10.1016/0028-3932(71)90067-4]
Paton, J. J., & Buonomano, D. V. (2018). The neural basis of timing: Distributed mechanisms for diverse functions. Neuron, 98(4), 687-705. [DOI:10.1016/j.neuron.2018.03.045] [PMID] [PMCID]
Penrose, L. S., & Raven, J. C. (1936). A new series of perceptual tests: Preliminary communication. British Journal of Medical Psychology, 16(2), 97-104. [DOI:10.1111/j.2044-8341.1936.tb00690.x]
Powers, A. R., Hillock-Dunn, A., & Wallace, M. T. (2016). Generalization of multisensory perceptual learning. Scientific Reports, 6(September 2015), 1-9. [DOI:10.1038/srep23374] [PMID] [PMCID]
Protopapa, F., Hayashi, M. J., Kulashekhar, S., van der Zwaag, W., Battistella, G., Murray, M. M., … Bueti, D. (2019). Chronotopic maps in the human supplementary motor area. PLoS Biology, 17(3), e3000026. [DOI:10.1371/journal.pbio.3000026] [PMID] [PMCID]
Rouzbahani, M., Motie, A. (2017). Effective connectivity estimation based on emotion EEG signal by granger causality and directed transfer function. Journal of Advances in Cognitive Sciences, 19(2), 1-18. [Link]
Shalbaf, R., Behnam, H., Sleigh, J. W., Steyn-Ross, D. A., & Steyn-Ross, M. L. (2015). Frontal-temporal synchronization of EEG signals quantified by order patterns crosses recurrence analysis during propofol anesthesia. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 23(3), 468-474. [DOI:10.1109/TNSRE.2014.2350537] [PMID]
Siman-Tov, T., Granot, R. Y., Shany, O., Singer, N., Hendler, T., & Gordon, C. R. (2019). Is there a prediction network? Meta-analytic evidence for a cortical-subcortical network likely subserving prediction. Neuroscience & Biobehavioral Reviews, 105, 262-275. [DOI:10.1016/j.neubiorev.2019.08.012] [PMID]
Soares, S., Atallah, B. V, & Paton, J. J. (2016). Midbrain dopamine neurons control judgment of time. Science, 354(6317), 1273-1277. [DOI:10.1126/science.aah5234] [PMID]
Stam, C. J., Nolte, G., & Daffertshofer, A. (2007). Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Human Brain Mapping, 28(11), 1178-1193. [DOI:10.1002/hbm.20346] [PMID] [PMCID]
Sugar, J., & Moser, M.-B. (2019). Episodic memory: Neuronal codes for what, where, and when. Hippocampus, 29(12), 1190-1205. [DOI:10.1002/hipo.23132] [PMID]
Tamburro, G., di Fronso, S., Robazza, C., Bertollo, M., & Comani, S. (2020). Modulation of brain functional connectivity and efficiency during an endurance cycling task: A source-level EEG and graph theory approach. Frontiers in Human Neuroscience, 14, 243. [DOI:10.3389/fnhum.2020.00243] [PMID] [PMCID]
Tinius, T. P. (2003). The integrated visual and auditory continuous performance test as a neuropsychological measure. Archives of Clinical Neuropsychology, 18(5), 439-454. [DOI:10.1016/S0887-6177(02)00144-0] [PMID]
Umbach, G., Kantak, P., Jacobs, J., Kahana, M., Pfeiffer, B. E., Sperling, M., et al. (2020). Time cells in the human Hippocampus and entorhinal cortex support episodic memory. Proceedings of the National Academy of Sciences, 117(45), 28463-28474. [DOI:10.1073/pnas.2013250117] [PMID] [PMCID]
Villalonga, M. B., Sussman, R. F., & Sekuler, R. (2021). Perceptual timing precision with vibrotactile, auditory, and multisensory stimuli. Attention, Perception, & Psychophysics, 83(5), 2267-2280. [DOI:10.3758/s13414-021-02254-9] [PMID]
Vinck, M., Oostenveld, R., Van Wingerden, M., Battaglia, F., & Pennartz, C. M. A. (2011). An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise, and sample-size bias. Neuroimage, 55(4), 1548-1565. [DOI:10.1016/j.neuroimage.2011.01.055] [PMID]
Wiener, M., Turkeltaub, P., & Coslett, H. B. (2010). The image of time: A voxel-wise meta-analysis. NeuroImage, 49(2), 1728-1740. [DOI:10.1016/j.neuroimage.2009.09.064] [PMID]
Wittmann, M., Simmons, A. N., Aron, J. L., & Paulus, M. P. (2010). Accumulation of neural activity in the posterior insula encodes the passage of time. Neuropsychologia, 48(10), 3110-3120. [DOI:10.1016/j.neuropsychologia.2010.06.023] [PMID] [PMCID]
Wright, B. A., Buonomano, D. V., Mahncke, H. W., & Merzenich, M. M. (1997). Learning and generalization of auditory temporal-interval discrimination in humans. Journal of Neuroscience, 17(10), 3956-3963. [DOI:10.1523/jneurosci.17-10-03956.1997] [PMID] [PMCID]
Wu, S., Shankar Gupta, D., Banerjee, A., Roy, D., & Piras, F. (2020). Editorial: Temporal structure of neural processes coupling sensory, motor and cognitive functions of the brain. Frontiers in Computational Neuroscience, 14, 73. [DOI:10.3389/fncom.2020.00073] [PMID] [PMCID]
Zippo, A. G., Della Rosa, P. A., Castiglioni, I., & Biella, G. E. M. (2018). Alternating dynamics of segregation and integration in human EEG functional networks during working-memory task. Neuroscience, 371, 191-206. [DOI:10.1016/j.neuroscience.2017.12.004] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2022/02/3 | Accepted: 2022/04/10 | Published: 2022/07/24
Received: 2022/02/3 | Accepted: 2022/04/10 | Published: 2022/07/24
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








 nirp.ir
nirp.ir