Volume 14, Issue 5 (September & October 2023)
BCN 2023, 14(5): 663-674 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rafiei S, Khodagholi F, Gholami Pourbadie H, Dargahi L, Motamedi F. Hepatic Acyl CoA Oxidase1 Inhibition Modifies Brain Lipids and Electrical Properties of Dentate Gyrus. BCN 2023; 14 (5) :663-674
URL: http://bcn.iums.ac.ir/article-1-2194-en.html
URL: http://bcn.iums.ac.ir/article-1-2194-en.html
Shahrbanoo Rafiei1 

 , Fariba Khodagholi2
, Fariba Khodagholi2 

 , Hamid Gholami Pourbadie3
, Hamid Gholami Pourbadie3 

 , Leila Dargahi1
, Leila Dargahi1 

 , Fereshteh Motamedi *
, Fereshteh Motamedi * 

 1
1


 , Fariba Khodagholi2
, Fariba Khodagholi2 

 , Hamid Gholami Pourbadie3
, Hamid Gholami Pourbadie3 

 , Leila Dargahi1
, Leila Dargahi1 

 , Fereshteh Motamedi *
, Fereshteh Motamedi * 

 1
1
1- Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Neurobiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Physiology and Pharmacology, Pasteur Institute of Iran, Tehran, Iran.
2- Neurobiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Physiology and Pharmacology, Pasteur Institute of Iran, Tehran, Iran.
Full-Text [PDF 1046 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Fatty acid degradation through the process of β-oxidation is a common metabolic pathway in both peroxisomes and mitochondria. However, certain mammalian fatty acids can be exclusively degraded by peroxisomes. They include dicarboxylic acids, bile acid intermediates, very-long-chain fatty acids (VLCFA), and phytanic acid (Poirier et al., 2006; Wanders & Waterham, 2006). While oxidation of polyunsaturated fatty acids (PUFAs) is slow in mitochondria, it is very fast in peroxisomes, (Hiltunen et al., 1986). Additionally, peroxisome β-oxidation contributes to the synthesis of the major brain PUFA, docosahexaenoic acid (DHA) (Ferdinandusse et al., 2001). Peroxisomes have a role in modulating mitochondrial regulatory factors, such as histone deacetylases 6 (HDAC6) and sirtuin-1 (SIRT1) (Rafiei et al., 2021). Acyl-CoA oxidase-1 is the rate-limiting enzyme of the peroxisome β-oxidation, which catalyzes the first step reaction. It can act on dicarboxylic acid, PUFAs, and saturated VLCFA without affecting branched-chain fatty acids or di or tri hydroxycholestanoic acids (bile acid intermediates). The deficiency of the Acyl CoA oxidase (ACOX1), an autosomal recessive disorder known as pseudo-neonatal adrenoleukodystrophy, is determined by the clinical presentation of hypotonia, seizures, and developmental retardation. Its biochemical diagnosis is the plasma accumulation of VLCFA C26:0 (Ferdinandusse et al., 2007). Some studies emphasize the decline of the peroxisome function during aging (Narayan et al., 2016). Moreover, in aged rodents, a decline of hepatic ACOX1 expression followed by altered brain fatty acid composition has been reported (Yang et al., 2014). In addition, growing evidence has suggested the relationship of peroxisome with age-related pathologies, such as neurodegeneration (Cipolla & Lodhi, 2017).
The dentate gyrus, involved in pattern separation, response decorrelation, spatial navigation, and engram formation, has been described as a more vulnerable hippocampal subregion to age-related processes (Small et al., 2004; Small et al., 2002). The brain has the highest lipid content after adipose tissue, and lipids comprise 50% of brain dry weight (Hamilton et al., 2007). Brain lipids have a crucial role in the development and maintenance of brain structure throughout life. By localizing proteins and allocating their function, brain lipids mainly organize the neuronal cell membrane (Simons & Toomre, 2000). Some studies have focused on the relationship between the brain lipid profile and its electrical properties. They have reported that dietary fat can influence these properties by remodeling brain lipid composition. Variations that occurred in the ratio of PUFAs to saturated fatty acids (P/S) in mice diets have made significant differences in membrane electrical properties of neurons in dorsal root ganglion (Scott et al., 1989). Lipid modification of the entorhinal cortex induced by diet has changed the physiological properties of neurons (Arsenault et al., 2012). Moreover, studies show that docosahexaenoic acid (DHA) accumulation improves cognition by altering the passive properties of EC neurons (Arsenault et al., 2011).
An acetylene derivative of fatty acids, 10, 12-tricosadiynoic acid (TDYA), was proposed as a specific ACOX1 inhibitor in a study in 2017, in which the inhibitory effect of TDYA was assessed in both in vitro and in vivo conditions on rodent hepatic ACOX1 (Zeng et al., 2017).
This study was conducted to examine the effect of inhibition of ACOX1 activity, a peroxisome β-oxidation enzyme, on cerebral lipid composition and electrophysiological properties of dentate gyrus granule cells (DG-GCs) in adult rats.
2. Materials and Methods
Animals
This study was conducted on the male Wistar rats (200-250 g) provided by a breeding colony in the Neuroscience Research Center (Tehran City, Iran). Rats in groups of four were housed in each cage with free access to water and chow. The standard cycle of 12 h light/12 h dark and a temperature of 22˚C-25˚C was set in the animal house.
Experimental design
The animals were divided into two groups and received an intragastric gavage administration of 100 μg/kg TDYA or olive oil as the drug's solvent for 25 consecutive days. Electrophysiological recordings and tissue collection for fatty acid profile analysis (brain hemispheres) were performed on the 25th day. For enzyme assay, five hours after the last administration of TDYA or vehicle, animals were decapitated, and hepatic tissue was removed. The collected tissues were quickly transferred to liquid nitrogen and then stored at -80˚C until use. For each experiment, the number of rats was as follows, enzymatic and fatty acid profile experiments (n=3 for each group) and electrophysiological experiments (n=4 for olive oil and n=6 for TDYA groups). In addition, 8 cells were used in electrophysiological experiments in olive oil and 6 cells in TDYA groups.
Acyl CoA oxidase (ACOX1) activity assay
ACOX1 activity assay was conducted based on the method of Cable et al. by measuring H2O2 production (Cablé et al., 1993). The reaction was started by adding 0.1 mM palmitoyl-coA into the reaction mixture containing liver homogenate, 0.01 mM flavin adenine dinucleotide (FAD), 0.082 mM 4-aminoantipyrine, 0.8 IU of horseradish peroxidase, 1.06 mM phenol, and 50 mM potassium phosphate, pH 8. Increasing the absorbance at 500 nm was recorded for 30 minutes and enzyme activity was calculated as nmol H2O2/min/mg protein by extinction coefficient of 480×105 mL mol-1 cm-1.
Fatty acid analysis
Brain lipids were extracted by two commonly used solvents, chloroform, and methanol, in a volume ratio of 2:1. The methyl ester fatty acids were derived from a BH3/methanol reagent and analyzed using Shimadzu Nexis 2030 gas chromatography with a detection system of flame-ionization. The chromatography column was Dikmacap-2330 (60 m×0.25 mm). Fatty acid separation was acquired in a two-stage temperature program by maintaining the initial temperature at 60˚C for 2 minutes and increasing it to 200˚C at a rate of 10˚C/min for 25 minutes. Then column reaches the final temperature of 240˚C at a rate of 5˚C/min and keeps it for 7 minutes. The chromatogram was analyzed by comparing the retention times of each peak with the peaks from the standard fatty acid methyl esters. Data were presented as the relative percentage by calculating the peak area ratio for each fatty acid to the total area from the rest of the peaks by the LabSolutions software.
In vitro whole cell patch clamp
Acute slice preparation
Transcardial perfusion in a deeply anesthetized rat (intraperitoneal ketamine/xylazine) was done with an ice-cold cutting solution containing 206 mM sucrose, 2.8 mM potassium chloride (KCl), 1.25 mM monosodium phosphate (NaH2PO4 ), 26 mM sodium bicarbonate (NaHCO3 ), 10 mM glucose, 1 mM calcium chloride (CaCl2), 2 mM magnesium sulfate (MgSO4), pH 7.4, oxygenated with molecular oxygen (O2) and carbon dioxide (CO2) (95%-5%). After rapidly removing the brain and immersing it into the cutting solution, the hippocampus was dissected out and sliced by a vibratome (752 HA, Campden Instruments Ltd, UK) to obtain 350 μm-thick slices that were then transferred into artificial cerebrospinal fluid (ACSF) solution (124 mM sodium chloride [NaCl]), 3 mM potassium chloride (KCl), 1.24 mM monosodium phosphate (NaH2PO4), 26 mM sodium bicarbonate (NaHCO3), 10 mM glucose, 2 mM CaCl2, 2 mM magnesium sulfate (MgSO4), pH 7.4, oxygenated with 95% molecular oxygen (O2), and 5% carbon dioxide (CO2) at 32°C for 30 minutes. In the recording process, a slice was located in a holding chamber of an upright microscope (Olympus; BX 51WI) stage and continuously superfused with the artificial cerebrospinal fluid (ACSF) solution at room temperature.
Whole-cell patch clamp recording
DG-GCs were visualized by infrared video imaging (Hamamatsu, ORSA, Japan) using a 40x water immersion objective lens. A PC-10 vertical puller (Narishige, Japan) was used for two-stage pulling of the borosilicate glass capillary (1.2 mm O.D×0.9 mm ID) to obtain 3-5 MΩ pipette resistance when filled with an intracellular solution. A whole-cell configuration was achieved by applying negative pressure to the gigaseal cell-attached statement. GCs were recorded by Multiclamp 700B amplifier (Axon Instruments, Foster City, CA) connected to an A/D converter of Digidata 1320 (Axon Instruments, Foster City, CA). Recordings with series resistance less than 25 MΩ and variation less than 20% during the experiment were selected for analysis. Current-clamp was done using an intracellular solution containing 140 mM K-Gluconate, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM magnesium chloride (MgCl2), 2 mM adenosine-5-triphosphate disodium salt (Na2-ATP), 1.1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 0.1 mM CaCl2, and 0.4 mM guanosine-5-triphosphate disodium salt (Na2-GTP) adjusted to pH 7.3 by potassium hydroxide (KOH), and the osmolality to 290 mOsm. The records were obtained at a sample rate of 20 kHz after applying a low-pass Bessel filter at 10 kHz and analyzed offline. The measured parameters were resting membrane potential (RMP), input resistance, time constant, action potential (AP) duration, rising time and decay time, firing frequency of neurons, AP peak amplitude, afterhyperpolarization (AHP) amplitude, and rheobase current.
Statistics
GraphPad Prism software, version 6.07 was used for analysis. All data were analyzed using student's t-test and expressed as Mean±SEM. The values of P<0.05 were statistically significant.
3. Results
Tricosadiynoic acid (TDYA) reduced hepatic ACOX1 activity
Data shown in Figure 1 shows ACOX1 activity in two comparing groups TDYA and olive oil as a control. The analysis revealed a significant 30% reduction in enzyme activity in the TDYA group (P<0.05) (Figure 1).

Inhibition of ACOX1 changed fatty acid composition in the brain
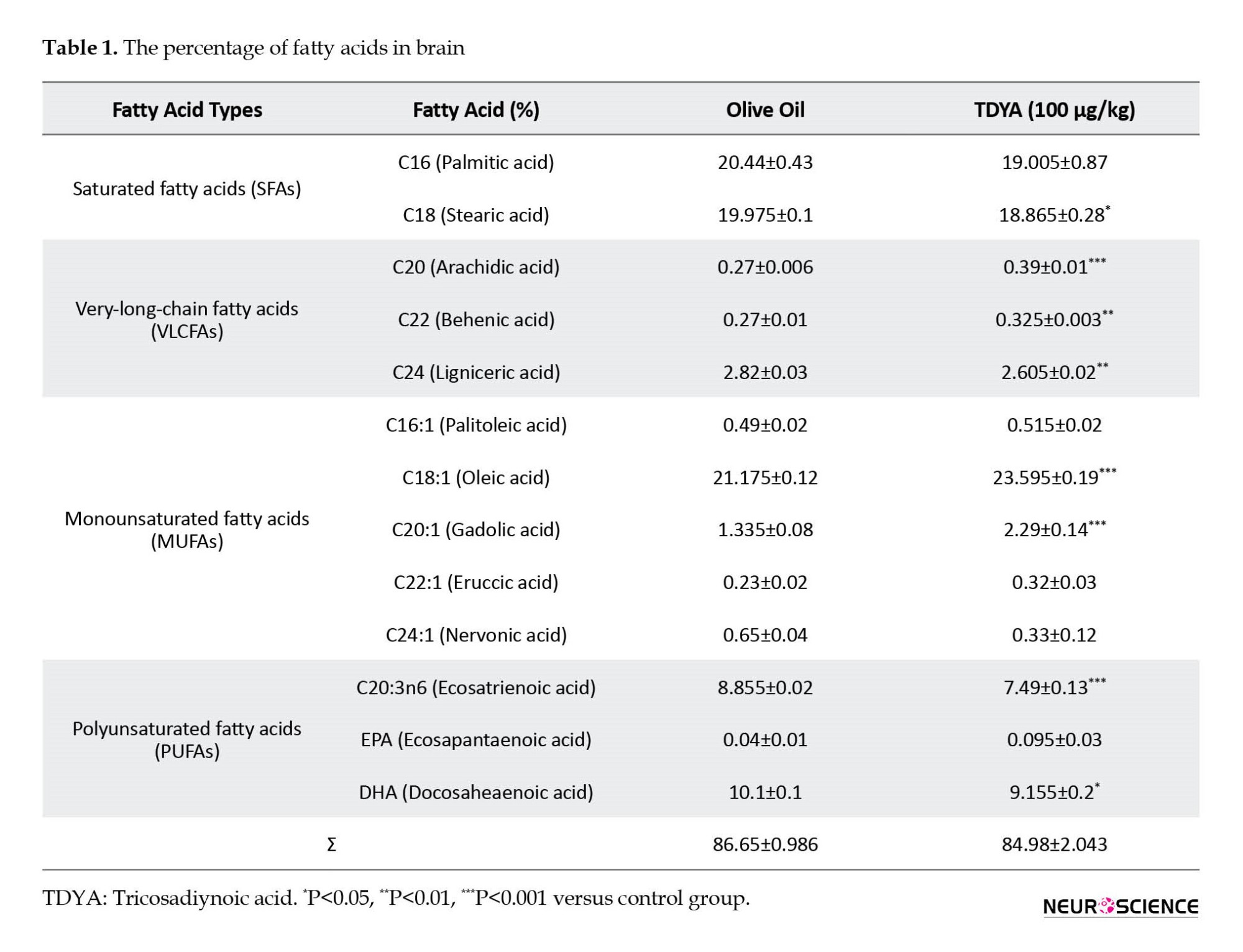
As shown in Table 1, only 13 types of fatty acids (of the total 31 detected types) were compared in our analyses (Table 1). Among all saturated fatty acids, C18:0 was significantly lower in the TDYA group (P<0.05). VLCFA levels (C20:0 and C22:0) were significantly higher in the TDYA (P<0.001, P<0.01), and in contrast, C24:0 was significantly decreased (P<0.01). The analyzed data for five monounsaturated fatty acids (MUFAs) revealed a significant increase for C18:1 and C20:1 (P<0.001, P<0.001). No significant difference was observed for C16:1, C22:1, and C24:1 compared to the control group. Among the three analyzed PUFAs, the TDYA group had a significant decrease in C20:3n6 and DHA (C22:6n3) levels (P<0.001, P<0.05).
Inhibition of hepatic ACOX1 resulted in hypo-excitability of dentate gyrus neuronal cells
After 25 days of TDYA treatment, significant differences in electrophysiological properties in DG-GCs were detected (Table 2). We found several differences in the passive properties of granule cells in the TDYA group compared to the control group. The student’s t-test analysis revealed a significant decrease in RMP of DG-GCs in the TDYA group (-76.98±1.5 mV) compared to the control group (-71.45±1.08 mV, P<0.01). In addition, the input resistance (Rin) of TDYA-treated rats (169.18±20.42 MΩ) compared to the control ones (270.66±23.37 MΩ) was significantly lower (P<0.01). Data analysis also indicated an increased time constant for the TDYA group (12.19±1.15 ms, P<0.05) compared to the control group (8.44±0.71 ms).
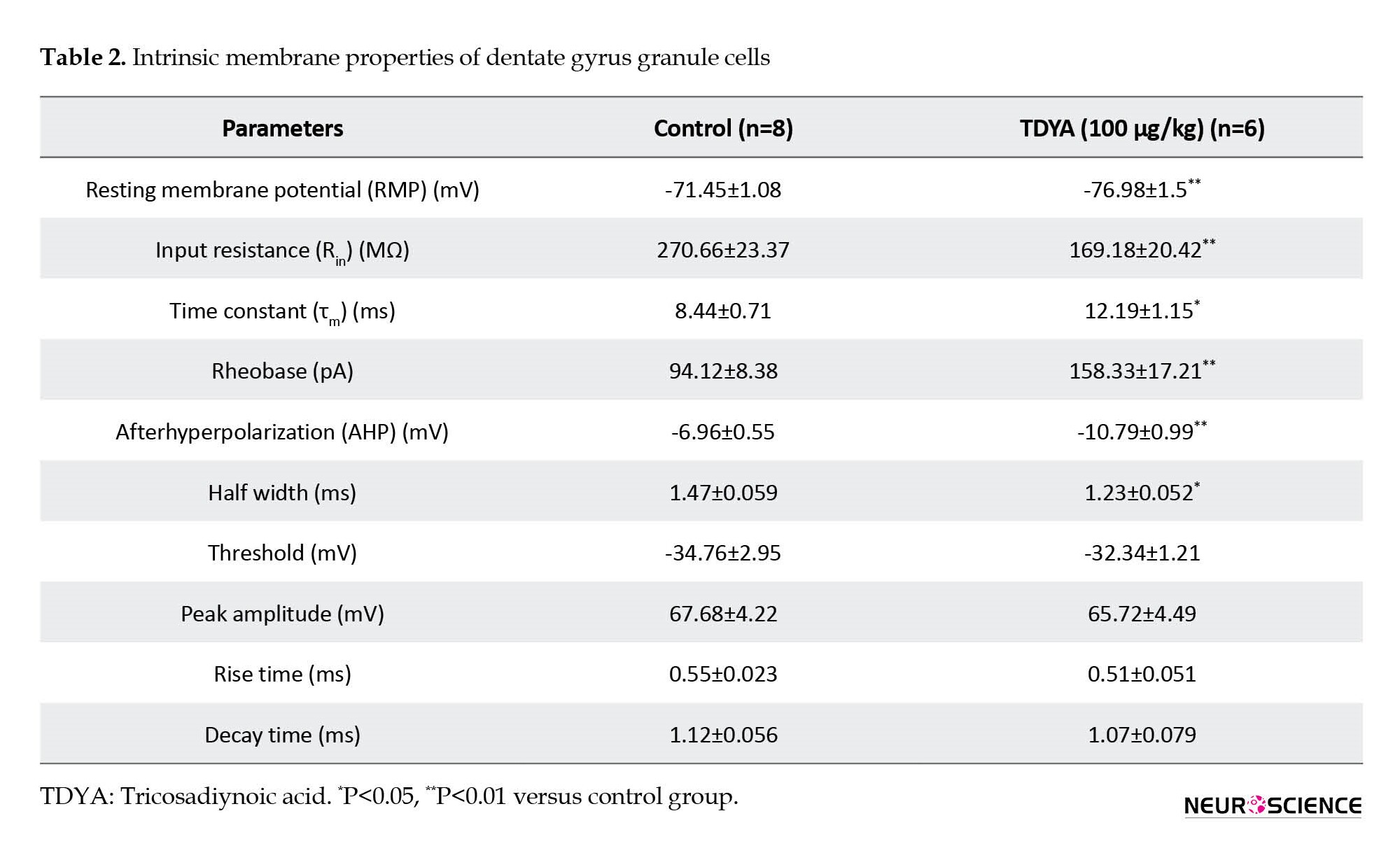
The current clamp was recorded to illustrate how the granule cell spike waveform can be affected by TDYA administration. TDYA had no significant effect on AP peak amplitude, threshold, and rise or decay slope (Table 2). As shown in Figure 2A, the granule cells of TDYA-treated rats fired at a much more depolarized current intensity than the control group. Further, DG-GCs from the TDYA group showed APs with a lower half-width (1.23±0.052 vs. 1.47±0.059 ms, P<0.05, Figure 2B), and higher amplitude for AHP compared to the control group (-10.8±0.99 mV vs. -6.96±0.55 mV, P<0.01, Figure 2C). Rheobase current was determined as the minimum current injected to trigger one AP during one second. As represented in Table 2 and Figure 2D, the rheobase current in the TDYA group was approximately 2-fold higher than the control group, indicating that TDYA induced hypo-excitability in DG-GCs.
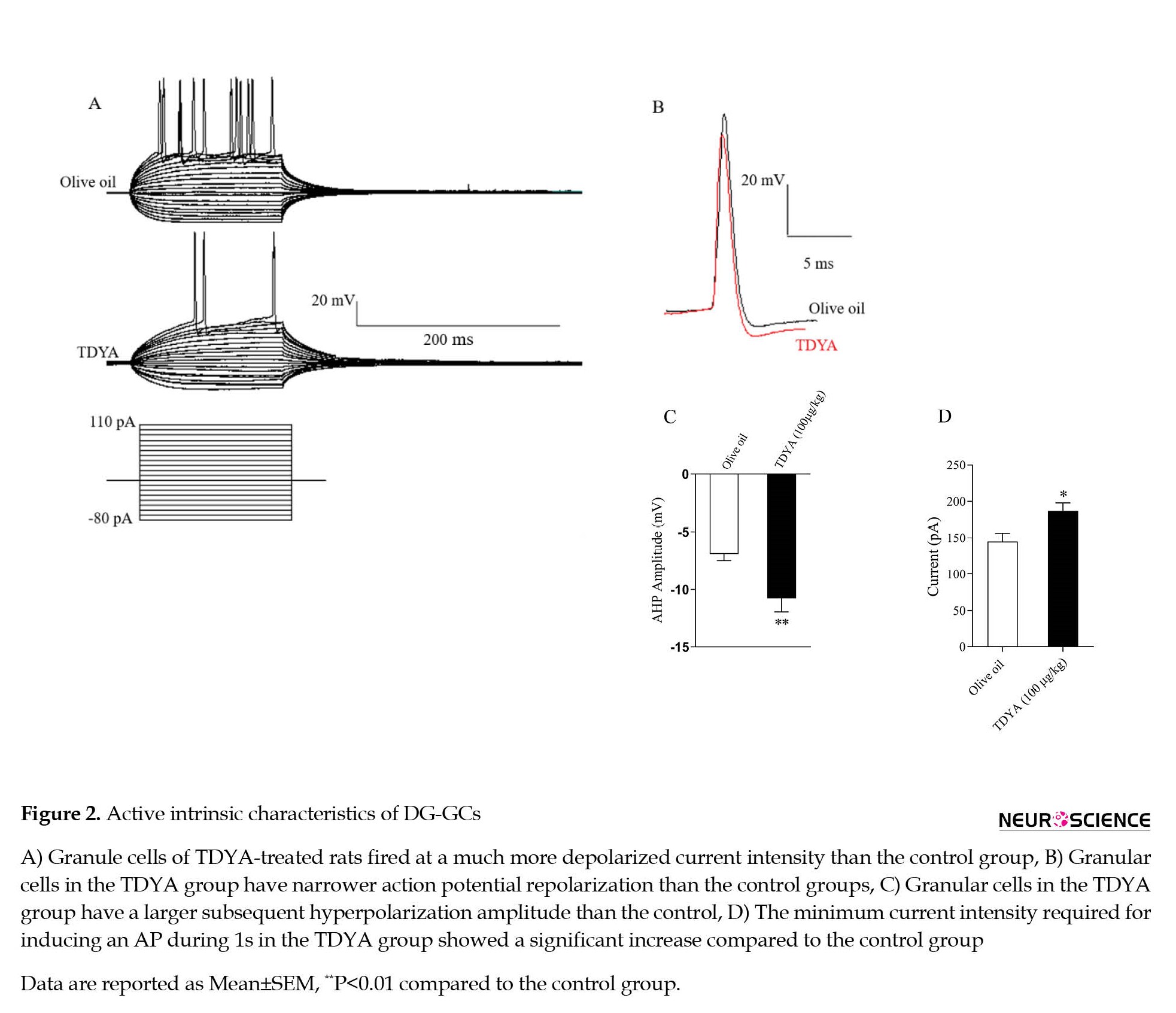
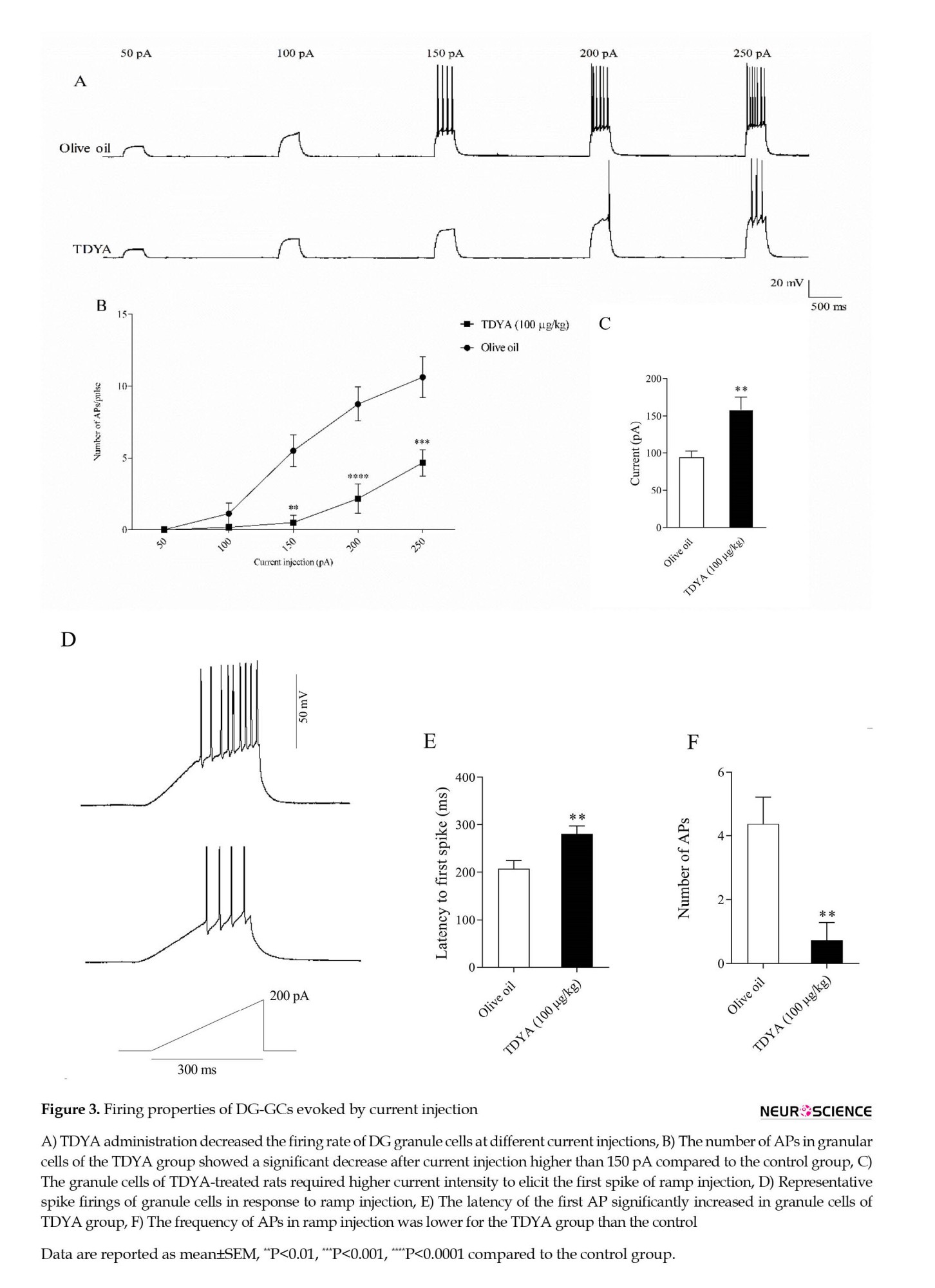
Figure 3A shows that TDYA administration decreased the firing rate of DG granule cells at different current injections, indicating that the granule cells in the TDYA group required a larger current to fire an AP. As shown in Figure 3B, current injection higher than 150 pA elicited lower AP numbers in the granule cells from TDYA-treated rats compared to the control group. Furthermore, the depolarizing current injected in the form of a ramp (200 pA, 300 ms) revealed a lower tendency of DG-GCs for firing in TDYA-treated rats (control 144.7±11.75 pA; TDYA 187.2±10.80 pA, P<0.05; Figure 3C, D). They also manifested a greater latency to fire the first AP (280.7±16.22 ms vs. 207.6±16.77 ms, P<0.01) and a lower number of APs (0.7143±0.56 vs. 4.375±0.84, P<0.01) compared to the control (Figure 3E, F). Together, these data confirm that TDYA administration leads to hypo-excitability in the DG-GCs.
4. Discussion
The present study is the first report on intrinsic membrane properties of DG-GCs after inhibition of hepatic ACOX1 and subsequent modified fatty acid profile in the brains of young rats. Inhibition of hepatic ACOX1 by TDYA altered the brain fatty acid composition, followed by decreased excitability of DG-GCs. We found a significant increase in MUFAs (C18:1, C20:1) and a reduction of PUFAs (C20:3n6 and C22:[DHA]) and C18:0 in the statistical analysis. For VLCFAs, a significant increase was found in C20:0 and C22:0 with a decrease in C24:0. A study published in 2014 reported that the expression of hepatic ACOX1 in old rats has been diminished and caused brain fatty acid alteration compared to younger ones (Yang et al., 2014). They saw an increase in VLCFAs C20:0, C24:0 and MUFAs C16:1, C18:1, C20:1, C22:1, C24:1, and a decrease in C18:0, C20:4, and DHA. We had the same trend for fatty acid differences, except for C16:1, C22:1, C24:0, and C24:1. Analysis of the cortical fatty acid profile in patients with two inherited peroxisomal disorders of Zellweger syndrome, a peroxisomal biogenesis disorder with absence or non-functional peroxisomes, and X-linked adrenoleukodystrophy, deficiency in the metabolism of VLCFAs, exhibited a decrease in C24:0, and C24:1 (Martinez, 1992). In addition, a significant decrement of an omega 6 PUFA, eicosatrienoic acid (C20:3n6) was observed, which is consistent with another study on aging (Carver et al., 2001). The levels of PUFAs decrease and MUFAs increase with aging (Lopez et al., 1995). Aberrant lipid metabolism and altered fatty acid composition in the brain have been suggested as an underlying mechanism for pathology involved in brain aging and certain neurodegenerative disorders, including AD and Parkinson’s disease (Cunnane et al., 2012; Fabelo et al., 2011). Some studies reported declined levels of C18:0 (stearic acid), arachidonic acid, and DHA in the brain of mild cognitive impairment and AD, with a significant enhancement of C18:1 (oleic acid), C16:0 (palmitic acid), and VLCFAs (Cunnane et al., 2012; Fraser et al., 2010). It can be concluded that ACOX1 inhibition in the present study may change brain lipid components at a younger age, similar to aging and AD.
Intrinsic membrane properties, categorized into passive and active characteristics of a neuron, can dictate neuronal excitability and affect its input/output gain (Kowalski et al., 2016). DG-GCs in TDYA-treated rats showed a more hyperpolarized RMP with a lower Rin and higher time constant (τm) than the control cells. Hyperpolarized RMP led to neuron hypo-excitability, and may be attributed to changed ion gradient or upregulation of new ion channels active in the resting state (Watari et al., 2013). Rin, and τm are two contributing factors in the integration of postsynaptic signals. Diminution of Rin can result from Rm reduction, which is inversely related to conductivity, and/or enhanced membrane surface. On the other hand, Rm and Cm, the parameters correlative to the cell surface, determine the τm (Young et al., 2009). The granule cells of the TDYA group manifested strongly enhanced τm values compared to the control. Since TDYA influenced Rin and τm in the opposite direction, it can be inferred that Cm was increased by the TDYA administration. Lipid composition can determine this value. In vitro, PUFA application has disclosed their differential effects on membrane capacitance. While eicosapentaenoic acid and arachidonic acid made capacitance increase, docosahexaenoic acid, linoleic acid, oleic acid did not change it (Ong et al., 2006). In analyzing the active properties of the granule cell membrane, no significant difference was observed in some AP parameters, including peak amplitude, threshold, rise time, and decay slope between groups. However, TDYA altered AP properties by enhancing rheobase current, decreasing AP duration, and increasing AHP amplitude with decreased AP firing frequency. The shape of the AP waveform has an essential effect on the firing frequency of a neuron. According to Ohm’s law (R=V/I), decreased Rin in TDYA-treated rats should reduce excitability. Therefore, enhanced rheobase current in this study was predictable. Variations in spike width can influence the gain of a neuron’s input/output relationship (Giese et al., 1998; Lin et al., 2014). Also, the AP duration (width) can be influenced by the rate of spike repolarization, which is commonly regulated by potassium voltage-gated channels (Bean, 2007). For instance, the enhanced density of these channels correlates with declined RMP, Rin, and AP duration observed with maturation for DG-GCs (Spigelman et al., 1992), which is similar to our results. Also, other potassium channels (fast delayed-rectifier K channels or BK channels) have been reported to control the AHP amplitude (Contet et al., 2016; Sesti et al., 2010). Therefore, alteration of potassium channels can be one of the possible mechanisms for ACOX1 inhibition in our study. The effect of fatty acids on ion channels, including potassium, has been subjected to numerous studies. Arachidonic acid, C14:0, C18:1, C18:3n3, and eicosatetraenoic acid have been reported to activate potassium currents. (Ordway et al., 1989). Furthermore, reports show different effects of PUFAs on different potassium channels. For instance, PUFAs have different effects on the voltage-gated potassium channels. While the KV1-4 channels are inactivated in the presence of PUFAs, these lipids can activate the slow rectifier K and BK channels, and increase their currents. (Börjesson & Elinder, 2011; Börjesson et al., 2008; Hoshi et al., 2013; Tian et al., 2016). In addition, both activation and inactivation of channels by PUFAs have been reported in the delayed rectifier potassium channels (Gavrilova-Ruch et al., 2007). It should be mentioned that their double bond, cis-geometry of double bond, and carboxyl group with negative charge have been proposed as the required properties of PUFAs in eliciting these effects (Boland et al., 2009; Elinder & Liin, 2017).
Studies have verified that the brain’s fatty acid components can be influenced by diet, aging, and age-related disorders (Chew et al., 2020; Ledesma et al., 2012; Pakiet et al., 2019). On the other hand, brain lipids are essential determinants of neuron electrical properties. A diet with a low-P/S ratio (supplied 2% of the calories as C18:2n6) affects the electrical properties of dorsal root ganglion cells by reducing AP duration, after hyperpolarization, Rin, and τm compared to a high-P/S diet (supplied 22% of the calories as C18:2n6) (Scott et al., 1989). Another study, focusing on the intrinsic properties of entorhinal cortex cells, indicated that canola/soybean oil in the diet decreased AP duration and postsynaptic response duration and enhanced the firing frequency (Arsenault et al., 2012). The ketogenic diet has displayed an anticonvulsant effect by increasing plasma PUFAs. This effect is mediated by modulating A-type potassium and transient Na channels (Tigerholm et al., 2012). It seems that ACOX1 inhibition may change brain activity by modifying its fatty acid ratios. Finally, according to the results of this study, peroxisomes and hepatic ACOX1 are involved in regulating brain fatty acid profile and electrophysiological properties of DG cells, possibly through potassium channels, which require detailed study in the future.
Ethical Considerations
Compliance with ethical guidelines
The Research and Ethics Committee of the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences approved all experiments (No.: IR.SBMU.MSP.REC.1396.168). Animals were treated according to the guide for the care and use of laboratory animals (National Institutes of Health Publication No. 80-23, revised 1996).
Funding
This article has been extracted from the PhD dissertation of Shahrbanoo Rafiei, approved by School of Medicine Shahid Beheshti University of Medical Sciences (Registration No.: 6). And it is part of the project funded by the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences and Iran National Science Foundation (INSF) (Fund No.: 96015430).
Authors' contributions
Conceptualization, supervision and funding acquisition: Fereshteh Motamedi, Fariba Khodagholi; Methodology: Fereshteh Motamedi, Fariba Khodagholi, Hamid Gholami Pourbadie and Leila Dargahi; Resources: Fereshteh Motamedi, Leila Dargahi and Fariba Khodagholi; Investigation and writing original draft: Shahrbanoo Rafiei; Review & editing: Fereshteh Motamedi, Shahrbanoo Rafiei, Hamid Gholami Pourbadie and Fariba Khodagholi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Shahid Beheshti University of Medical Sciences and Iran National Science Foundation (INSF) for financial support.
References
Arsenault, D., Julien, C., Chen, C. T., Bazinet, R. P., & Calon, F. (2012). Dietary intake of unsaturated fatty acids modulates physiological properties of entorhinal cortex neurons in mice. Journal of Neurochemistry, 122(2), 427-443. [DOI:10.1111/j.1471-4159.2012.07772.x] [PMID]
Arsenault, D., Julien, C., Tremblay, C., & Calon, F. (2011). DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3xTg-AD mice. PloS One, 6(2), e17397. [DOI:10.1371/journal.pone.0017397] [PMID]
Bean, B. P. (2007). The action potential in mammalian central neurons. Nature Reviews. Neuroscience, 8(6), 451-465. [DOI:10.1038/nrn2148] [PMID]
Boland, L. M., Drzewiecki, M. M., Timoney, G., & Casey, E. (2009). Inhibitory effects of polyunsaturated fatty acids on Kv4/KChIP potassium channels. American Journal of Physiology-Cell Physiology, 296(5), C1003-C1014. [DOI:10.1152/ajpcell.00474.2008] [PMID]
Börjesson, S. I., & Elinder, F. (2011). An electrostatic potassium channel opener targeting the final voltage sensor transition. Journal of General Physiology, 137(6), 563-577. [DOI:10.1085/jgp.201110599] [PMID]
Börjesson, S. I., Hammarström, S., & Elinder, F. (2008). Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids. Biophysical Journal, 95(5), 2242-2253. [DOI:10.1529/biophysj.108.130757] [PMID]
Cablé, S., Kedinger, M., & Dauça, M. (1993). Peroxisomes and peroxisomal enzymes along the crypt-villus axis of the rat intestine. Differentiation, 54(2), 99-108. [PMID]
Carver, J. D., Benford, V. J., Han, B., & Cantor, A. B. (2001). The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Research Bulletin, 56(2), 79-85. [DOI:10.1016/S0361-9230(01)00551-2] [PMID]
Chew, H., Solomon, V. A., & Fonteh, A. N. (2020). Involvement of lipids in Alzheimer’s disease pathology and potential therapies. Frontiers in Physiology, 11, 598. [DOI:10.3389/fphys.2020.00598] [PMID]
Cipolla, C. M., & Lodhi, I. J. (2017). Peroxisomal dysfunction in age-related diseases. Trends in Endocrinology & Metabolism, 28(4), 297-308. [DOI:10.1016/j.tem.2016.12.003] [PMID]
Contet, C., Goulding, S. P., Kuljis, D. A., & Barth, A. (2016). BK channels in the central nervous system. International Review of Neurobiology, 128, 281-342. [DOI:10.1016/bs.irn.2016.04.001] [PMID]
Cunnane, S. C., Schneider, J. A., Tangney, C., Tremblay-Mercier, J., Fortier, M., & Bennett, D. A., et al. (2012). Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s Disease, 29(3), 691-697. [DOI:10.3233/JAD-2012-110629] [PMID]
Elinder, F., & Liin, S. I. (2017). Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Frontiers in Physiology, 8, 43. [DOI:10.3389/fphys.2017.00043] [PMID]
Fabelo, N., Martín, V., Santpere, G., Marín, R., Torrent, L., & Ferrer, I., et al. (2011). Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Molecular Medicine, 17(9-10), 1107-1118. [DOI:10.2119/molmed.2011.00119] [PMID]
Ferdinandusse, S., Denis, S., Hogenhout, E. M., Koster, J., van Roermund, C. W., & IJlst, L., et al. (2007). Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Human Mutation, 28(9), 904-912. [DOI:10.1002/humu.20535] [PMID]
Ferdinandusse, S., Denis, S., Mooijer, P. A., Zhang, Z., Reddy, J. K., & Spector, A. A., et al. (2001). Identification of the peroxisomal β-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. Journal of Lipid Research, 42(12), 1987-1995. [DOI:10.1016/S0022-2275(20)31527-3] [PMID]
Fraser, T., Tayler, H., & Love, S. (2010). Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochemical Research, 35(3), 503-513. [DOI:10.1007/s11064-009-0087-5] [PMID]
Gavrilova-Ruch, O., Schönherr, R., & Heinemann, S. H. (2007).Activation of hEAG1 potassium channels by arachidonic acid. Pflugers Archiv : European Journal of Physiology, 453(6), 891-903. [DOI:10.1007/s00424-006-0173-3] [PMID]
Giese, K. P., Storm, J. F., Reuter, D., Fedorov, N. B., Shao, L. R., & Leicher, T., et al. (1998). Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvβ1. 1-deficient mice with impaired learning. Learning & Memory, 5(4), 257-273. [DOI:10.1101/lm.5.4.257]
Hamilton, J. A., Hillard, C. J., Spector, A. A., & Watkins, P. A. (2007). Brain uptake and utilization of fatty acids, lipids and lipoproteins: Application to neurological disorders. Journal of Molecular Neuroscience, 33(1), 2-11. [DOI:10.1007/s12031-007-0060-1] [PMID]
Hiltunen, J. K., Kärki, T., Hassinen, I. E., & Osmundsen, H. (1986). Beta-Oxidation of polyunsaturated fatty acids by rat liver peroxisomes. A role for 2, 4-dienoyl-coenzyme A reductase in peroxisomal beta-oxidation.The Journal of Biological Chemistry, 261(35), 16484–16493. [DOI:10.1016/S0021-9258(18)66592-5] [PMID]
Hoshi, T., Xu, R., Hou, S., Heinemann, S. H., & Tian, Y. (2013). A point mutation in the human Slo1 channel that impairs its sensitivity to omega-3 docosahexaenoic acid. The Journal of General Physiology, 142(5), 507–522. [DOI:10.1085/jgp.201311061] [PMID]
Kowalski, J., Gan, J., Jonas, P., & Pernía-Andrade, A. J. (2016). Intrinsic membrane properties determine hippocampal differential firing pattern in vivo in anesthetized rats. Hippocampus, 26(5), 668-682. [DOI:10.1002/hipo.22550] [PMID]
Ledesma, M. D., Martin, M. G., & Dotti, C. G. (2012). Lipid changes in the aged brain: Effect on synaptic function and neuronal survival. Progress in Lipid Research, 51(1), 23–35. [DOI:10.1016/j.plipres.2011.11.004] [PMID]
Lin, M., Hatcher, J. T., Wurster, R. D., Chen, Q. H., & Cheng, Z. J. (2014). Characteristics of single large-conductance Ca2+-activated K+ channels and their regulation of action potentials and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus. American Journal of Physiology. Cell Physiology, 306(2), C152–C166. [DOI:10.1152/ajpcell.00423.2012] [PMID]
López, G. H., Ilincheta de Boschero, M. G., Castagnet, P. I., & Giusto, N. M. (1995). Age-associated changes in the content and fatty acid composition of brain glycerophospholipids. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 112(2), 331-343. [DOI:10.1016/0305-0491(95)00079-8] [PMID]
Martinez, M. (1992). Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders. Brain Research, 583(1-2), 171-182. [DOI:10.1016/S0006-8993(10)80021-6]
Narayan, V., Ly, T., Pourkarimi, E., Murillo, A. B., Gartner, A., & Lamond, A. I., et al. (2016). Deep proteome analysis identifies age-related processes in C. elegans. Cell Systems, 3(2), 144-159. [DOI:10.1016/j.cels.2016.06.011] [PMID]
Ong, W. L., Jiang, B., Tang, N., Ling, S. F., Yeo, J. F., & Wei, S., et al. (2006). Differential effects of polyunsaturated fatty acids on membrane capacitance and exocytosis in rat pheochromocytoma-12 cells. Neurochemical Research, 31(1), 41-48. [PMID]
Ordway, R. W., Walsh, J. V., Jr, & Singer, J. J. (1989). Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science, 244(4909), 1176-1179. [DOI:10.1126/science.2471269] [PMID]
Pakiet, A., Jakubiak, A., Czumaj, A., Sledzinski, T., & Mika, A. (2019). The effect of western diet on mice brain lipid composition. Nutrition & Metabolism, 16, 81. [DOI:10.1186/s12986-019-0401-4] [PMID]
Poirier, Y., Antonenkov, V. D., Glumoff, T., & Hiltunen, J. K. (2006). Peroxisomal β-oxidation-a metabolic pathway with multiple functions. Biochimica et Biophysica Acta, 1763(12), 1413-1426. [DOI:10.1016/j.bbamcr.2006.08.034] [PMID]
Rafiei, Sh., Khodagholi, F., Motamedi, F., & Dargahi, L. (2021). Peroxisome biogenesis factor 5 (PEX5) controls HDAC6 and SIRT1 expression and modulates mitochondrial biogenesis in rat dorsal hippocampus. Physiology and Pharmacology, 25(4), 341-352 [DOI:10.52547/phypha.25.4.10]
Scott, B., Lew, J., Clandinin, M., & Cinader, B. (1989). Dietary fat influences electric membrane properties of neurons in cell culture. Cellular and Molecular Neurobiology, 9(1), 105-113. [DOI:10.1007/BF00711447] [PMID]
Sesti, F., Liu, S., & Cai, S. Q. (2010). Oxidation of potassium channels by ROS: A general mechanism of aging and neurodegeneration? Trends in Cell Biology, 20(1), 45-51. [DOI:10.1016/j.tcb.2009.09.008] [PMID]
Simons, K., & Toomre, D. (2000). Lipid rafts and signal transduction. Nature Reviews. Molecular Cell Biology, 1(1), 31-39. [DOI:10.1038/35036052] [PMID]
Small, S. A., Chawla, M. K., Buonocore, M., Rapp, P. R., & Barnes, C. A. (2004). Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proceedings of the National Academy of Sciences of the United States of America, 101(18), 7181–7186. [DOI:10.1073/pnas.0400285101] [PMID]
Small, S. A., Tsai, W. Y., DeLaPaz, R., Mayeux, R., & Stern, Y. (2002). Imaging hippocampal function across the human life span: is memory decline normal or not? Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 51(3), 290-295. [DOI:10.1002/ana.10105] [PMID]
Spigelman, I., Zhang, L., & Carlen, P. L. (1992). Patch-clamp study of postnatal development of CA1 neurons in rat hippocampal slices: membrane excitability and K+ currents. Journal of Neurophysiology, 68(1), 55-69. [DOI:10.1152/jn.1992.68.1.55] [PMID]
Tian, Y., Aursnes, M., Hansen, T. V., Tungen, J. E., Galpin, J. D., & Leisle, L., et al. (2016). Atomic determinants of BK channel activation by polyunsaturated fatty acids. Proceedings of the National Academy of Sciences of the United States of America, 113(48), 13905–13910. [DOI:10.1073/pnas.1615562113] [PMID]
Tigerholm, J., Börjesson, S. I., Lundberg, L., Elinder, F., & Fransén, E. (2012). Dampening of hyperexcitability in CA1 pyramidal neurons by polyunsaturated fatty acids acting on voltage-gated ion channels. PloS One, 7(9), e44388. [DOI:10.1371/journal.pone.0044388] [PMID]
Wanders, R. J., & Waterham, H. R. (2006). Biochemistry of mammalian peroxisomes revisited. Annual Review of Biochemistry, 75, 295–332. [DOI:10.1146/annurev.biochem.74.082803.133329] [PMID]
Watari, H., Tose, A. J., & Bosma, M. M. (2013). Hyperpolarization of resting membrane potential causes retraction of spontaneous transients during mouse embryonic circuit development. The Journal of physiology, 591(4), 973-983. [DOI:10.1113/jphysiol.2012.244954] [PMID]
Yang, L., Zhang, Y., Wang, S., Zhang, W., & Shi, R. (2014). Decreased liver peroxisomal β-oxidation accompanied by changes in brain fatty acid composition in aged rats. Neurological Sciences, 35(2), 289-293. [DOI:10.1007/s10072-013-1509-3] [PMID]
Young, C. C., Stegen, M., Bernard, R., Müller, M., Bischofberger, J., & Veh, R. W., et al. (2009). Upregulation of inward rectifier K+ (Kir2) channels in dentate gyrus granule cells in temporal lobe epilepsy. The Journal of Physiology, 587(17), 4213-4233. [DOI:10.1113/jphysiol.2009.170746] [PMID]
Zeng, J., Deng, S., Wang, Y., Li, P., Tang, L., & Pang, Y. (2017). Specific inhibition of Acyl-CoA oxidase-1 by an acetylenic acid improves hepatic lipid and reactive oxygen species (ROS) metabolism in rats fed a high fat diet. Journal of Biological Chemistry, 292(9), 3800-3809. [DOI:10.1074/jbc.M116.763532] [PMID]
Fatty acid degradation through the process of β-oxidation is a common metabolic pathway in both peroxisomes and mitochondria. However, certain mammalian fatty acids can be exclusively degraded by peroxisomes. They include dicarboxylic acids, bile acid intermediates, very-long-chain fatty acids (VLCFA), and phytanic acid (Poirier et al., 2006; Wanders & Waterham, 2006). While oxidation of polyunsaturated fatty acids (PUFAs) is slow in mitochondria, it is very fast in peroxisomes, (Hiltunen et al., 1986). Additionally, peroxisome β-oxidation contributes to the synthesis of the major brain PUFA, docosahexaenoic acid (DHA) (Ferdinandusse et al., 2001). Peroxisomes have a role in modulating mitochondrial regulatory factors, such as histone deacetylases 6 (HDAC6) and sirtuin-1 (SIRT1) (Rafiei et al., 2021). Acyl-CoA oxidase-1 is the rate-limiting enzyme of the peroxisome β-oxidation, which catalyzes the first step reaction. It can act on dicarboxylic acid, PUFAs, and saturated VLCFA without affecting branched-chain fatty acids or di or tri hydroxycholestanoic acids (bile acid intermediates). The deficiency of the Acyl CoA oxidase (ACOX1), an autosomal recessive disorder known as pseudo-neonatal adrenoleukodystrophy, is determined by the clinical presentation of hypotonia, seizures, and developmental retardation. Its biochemical diagnosis is the plasma accumulation of VLCFA C26:0 (Ferdinandusse et al., 2007). Some studies emphasize the decline of the peroxisome function during aging (Narayan et al., 2016). Moreover, in aged rodents, a decline of hepatic ACOX1 expression followed by altered brain fatty acid composition has been reported (Yang et al., 2014). In addition, growing evidence has suggested the relationship of peroxisome with age-related pathologies, such as neurodegeneration (Cipolla & Lodhi, 2017).
The dentate gyrus, involved in pattern separation, response decorrelation, spatial navigation, and engram formation, has been described as a more vulnerable hippocampal subregion to age-related processes (Small et al., 2004; Small et al., 2002). The brain has the highest lipid content after adipose tissue, and lipids comprise 50% of brain dry weight (Hamilton et al., 2007). Brain lipids have a crucial role in the development and maintenance of brain structure throughout life. By localizing proteins and allocating their function, brain lipids mainly organize the neuronal cell membrane (Simons & Toomre, 2000). Some studies have focused on the relationship between the brain lipid profile and its electrical properties. They have reported that dietary fat can influence these properties by remodeling brain lipid composition. Variations that occurred in the ratio of PUFAs to saturated fatty acids (P/S) in mice diets have made significant differences in membrane electrical properties of neurons in dorsal root ganglion (Scott et al., 1989). Lipid modification of the entorhinal cortex induced by diet has changed the physiological properties of neurons (Arsenault et al., 2012). Moreover, studies show that docosahexaenoic acid (DHA) accumulation improves cognition by altering the passive properties of EC neurons (Arsenault et al., 2011).
An acetylene derivative of fatty acids, 10, 12-tricosadiynoic acid (TDYA), was proposed as a specific ACOX1 inhibitor in a study in 2017, in which the inhibitory effect of TDYA was assessed in both in vitro and in vivo conditions on rodent hepatic ACOX1 (Zeng et al., 2017).
This study was conducted to examine the effect of inhibition of ACOX1 activity, a peroxisome β-oxidation enzyme, on cerebral lipid composition and electrophysiological properties of dentate gyrus granule cells (DG-GCs) in adult rats.
2. Materials and Methods
Animals
This study was conducted on the male Wistar rats (200-250 g) provided by a breeding colony in the Neuroscience Research Center (Tehran City, Iran). Rats in groups of four were housed in each cage with free access to water and chow. The standard cycle of 12 h light/12 h dark and a temperature of 22˚C-25˚C was set in the animal house.
Experimental design
The animals were divided into two groups and received an intragastric gavage administration of 100 μg/kg TDYA or olive oil as the drug's solvent for 25 consecutive days. Electrophysiological recordings and tissue collection for fatty acid profile analysis (brain hemispheres) were performed on the 25th day. For enzyme assay, five hours after the last administration of TDYA or vehicle, animals were decapitated, and hepatic tissue was removed. The collected tissues were quickly transferred to liquid nitrogen and then stored at -80˚C until use. For each experiment, the number of rats was as follows, enzymatic and fatty acid profile experiments (n=3 for each group) and electrophysiological experiments (n=4 for olive oil and n=6 for TDYA groups). In addition, 8 cells were used in electrophysiological experiments in olive oil and 6 cells in TDYA groups.
Acyl CoA oxidase (ACOX1) activity assay
ACOX1 activity assay was conducted based on the method of Cable et al. by measuring H2O2 production (Cablé et al., 1993). The reaction was started by adding 0.1 mM palmitoyl-coA into the reaction mixture containing liver homogenate, 0.01 mM flavin adenine dinucleotide (FAD), 0.082 mM 4-aminoantipyrine, 0.8 IU of horseradish peroxidase, 1.06 mM phenol, and 50 mM potassium phosphate, pH 8. Increasing the absorbance at 500 nm was recorded for 30 minutes and enzyme activity was calculated as nmol H2O2/min/mg protein by extinction coefficient of 480×105 mL mol-1 cm-1.
Fatty acid analysis
Brain lipids were extracted by two commonly used solvents, chloroform, and methanol, in a volume ratio of 2:1. The methyl ester fatty acids were derived from a BH3/methanol reagent and analyzed using Shimadzu Nexis 2030 gas chromatography with a detection system of flame-ionization. The chromatography column was Dikmacap-2330 (60 m×0.25 mm). Fatty acid separation was acquired in a two-stage temperature program by maintaining the initial temperature at 60˚C for 2 minutes and increasing it to 200˚C at a rate of 10˚C/min for 25 minutes. Then column reaches the final temperature of 240˚C at a rate of 5˚C/min and keeps it for 7 minutes. The chromatogram was analyzed by comparing the retention times of each peak with the peaks from the standard fatty acid methyl esters. Data were presented as the relative percentage by calculating the peak area ratio for each fatty acid to the total area from the rest of the peaks by the LabSolutions software.
In vitro whole cell patch clamp
Acute slice preparation
Transcardial perfusion in a deeply anesthetized rat (intraperitoneal ketamine/xylazine) was done with an ice-cold cutting solution containing 206 mM sucrose, 2.8 mM potassium chloride (KCl), 1.25 mM monosodium phosphate (NaH2PO4 ), 26 mM sodium bicarbonate (NaHCO3 ), 10 mM glucose, 1 mM calcium chloride (CaCl2), 2 mM magnesium sulfate (MgSO4), pH 7.4, oxygenated with molecular oxygen (O2) and carbon dioxide (CO2) (95%-5%). After rapidly removing the brain and immersing it into the cutting solution, the hippocampus was dissected out and sliced by a vibratome (752 HA, Campden Instruments Ltd, UK) to obtain 350 μm-thick slices that were then transferred into artificial cerebrospinal fluid (ACSF) solution (124 mM sodium chloride [NaCl]), 3 mM potassium chloride (KCl), 1.24 mM monosodium phosphate (NaH2PO4), 26 mM sodium bicarbonate (NaHCO3), 10 mM glucose, 2 mM CaCl2, 2 mM magnesium sulfate (MgSO4), pH 7.4, oxygenated with 95% molecular oxygen (O2), and 5% carbon dioxide (CO2) at 32°C for 30 minutes. In the recording process, a slice was located in a holding chamber of an upright microscope (Olympus; BX 51WI) stage and continuously superfused with the artificial cerebrospinal fluid (ACSF) solution at room temperature.
Whole-cell patch clamp recording
DG-GCs were visualized by infrared video imaging (Hamamatsu, ORSA, Japan) using a 40x water immersion objective lens. A PC-10 vertical puller (Narishige, Japan) was used for two-stage pulling of the borosilicate glass capillary (1.2 mm O.D×0.9 mm ID) to obtain 3-5 MΩ pipette resistance when filled with an intracellular solution. A whole-cell configuration was achieved by applying negative pressure to the gigaseal cell-attached statement. GCs were recorded by Multiclamp 700B amplifier (Axon Instruments, Foster City, CA) connected to an A/D converter of Digidata 1320 (Axon Instruments, Foster City, CA). Recordings with series resistance less than 25 MΩ and variation less than 20% during the experiment were selected for analysis. Current-clamp was done using an intracellular solution containing 140 mM K-Gluconate, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM magnesium chloride (MgCl2), 2 mM adenosine-5-triphosphate disodium salt (Na2-ATP), 1.1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 0.1 mM CaCl2, and 0.4 mM guanosine-5-triphosphate disodium salt (Na2-GTP) adjusted to pH 7.3 by potassium hydroxide (KOH), and the osmolality to 290 mOsm. The records were obtained at a sample rate of 20 kHz after applying a low-pass Bessel filter at 10 kHz and analyzed offline. The measured parameters were resting membrane potential (RMP), input resistance, time constant, action potential (AP) duration, rising time and decay time, firing frequency of neurons, AP peak amplitude, afterhyperpolarization (AHP) amplitude, and rheobase current.
Statistics
GraphPad Prism software, version 6.07 was used for analysis. All data were analyzed using student's t-test and expressed as Mean±SEM. The values of P<0.05 were statistically significant.
3. Results
Tricosadiynoic acid (TDYA) reduced hepatic ACOX1 activity
Data shown in Figure 1 shows ACOX1 activity in two comparing groups TDYA and olive oil as a control. The analysis revealed a significant 30% reduction in enzyme activity in the TDYA group (P<0.05) (Figure 1).

Inhibition of ACOX1 changed fatty acid composition in the brain
As shown in Table 1, only 13 types of fatty acids (of the total 31 detected types) were compared in our analyses (Table 1). Among all saturated fatty acids, C18:0 was significantly lower in the TDYA group (P<0.05). VLCFA levels (C20:0 and C22:0) were significantly higher in the TDYA (P<0.001, P<0.01), and in contrast, C24:0 was significantly decreased (P<0.01). The analyzed data for five monounsaturated fatty acids (MUFAs) revealed a significant increase for C18:1 and C20:1 (P<0.001, P<0.001). No significant difference was observed for C16:1, C22:1, and C24:1 compared to the control group. Among the three analyzed PUFAs, the TDYA group had a significant decrease in C20:3n6 and DHA (C22:6n3) levels (P<0.001, P<0.05).

Inhibition of hepatic ACOX1 resulted in hypo-excitability of dentate gyrus neuronal cells
After 25 days of TDYA treatment, significant differences in electrophysiological properties in DG-GCs were detected (Table 2). We found several differences in the passive properties of granule cells in the TDYA group compared to the control group. The student’s t-test analysis revealed a significant decrease in RMP of DG-GCs in the TDYA group (-76.98±1.5 mV) compared to the control group (-71.45±1.08 mV, P<0.01). In addition, the input resistance (Rin) of TDYA-treated rats (169.18±20.42 MΩ) compared to the control ones (270.66±23.37 MΩ) was significantly lower (P<0.01). Data analysis also indicated an increased time constant for the TDYA group (12.19±1.15 ms, P<0.05) compared to the control group (8.44±0.71 ms).

The current clamp was recorded to illustrate how the granule cell spike waveform can be affected by TDYA administration. TDYA had no significant effect on AP peak amplitude, threshold, and rise or decay slope (Table 2). As shown in Figure 2A, the granule cells of TDYA-treated rats fired at a much more depolarized current intensity than the control group. Further, DG-GCs from the TDYA group showed APs with a lower half-width (1.23±0.052 vs. 1.47±0.059 ms, P<0.05, Figure 2B), and higher amplitude for AHP compared to the control group (-10.8±0.99 mV vs. -6.96±0.55 mV, P<0.01, Figure 2C). Rheobase current was determined as the minimum current injected to trigger one AP during one second. As represented in Table 2 and Figure 2D, the rheobase current in the TDYA group was approximately 2-fold higher than the control group, indicating that TDYA induced hypo-excitability in DG-GCs.

Figure 3A shows that TDYA administration decreased the firing rate of DG granule cells at different current injections, indicating that the granule cells in the TDYA group required a larger current to fire an AP. As shown in Figure 3B, current injection higher than 150 pA elicited lower AP numbers in the granule cells from TDYA-treated rats compared to the control group. Furthermore, the depolarizing current injected in the form of a ramp (200 pA, 300 ms) revealed a lower tendency of DG-GCs for firing in TDYA-treated rats (control 144.7±11.75 pA; TDYA 187.2±10.80 pA, P<0.05; Figure 3C, D). They also manifested a greater latency to fire the first AP (280.7±16.22 ms vs. 207.6±16.77 ms, P<0.01) and a lower number of APs (0.7143±0.56 vs. 4.375±0.84, P<0.01) compared to the control (Figure 3E, F). Together, these data confirm that TDYA administration leads to hypo-excitability in the DG-GCs.

4. Discussion
The present study is the first report on intrinsic membrane properties of DG-GCs after inhibition of hepatic ACOX1 and subsequent modified fatty acid profile in the brains of young rats. Inhibition of hepatic ACOX1 by TDYA altered the brain fatty acid composition, followed by decreased excitability of DG-GCs. We found a significant increase in MUFAs (C18:1, C20:1) and a reduction of PUFAs (C20:3n6 and C22:[DHA]) and C18:0 in the statistical analysis. For VLCFAs, a significant increase was found in C20:0 and C22:0 with a decrease in C24:0. A study published in 2014 reported that the expression of hepatic ACOX1 in old rats has been diminished and caused brain fatty acid alteration compared to younger ones (Yang et al., 2014). They saw an increase in VLCFAs C20:0, C24:0 and MUFAs C16:1, C18:1, C20:1, C22:1, C24:1, and a decrease in C18:0, C20:4, and DHA. We had the same trend for fatty acid differences, except for C16:1, C22:1, C24:0, and C24:1. Analysis of the cortical fatty acid profile in patients with two inherited peroxisomal disorders of Zellweger syndrome, a peroxisomal biogenesis disorder with absence or non-functional peroxisomes, and X-linked adrenoleukodystrophy, deficiency in the metabolism of VLCFAs, exhibited a decrease in C24:0, and C24:1 (Martinez, 1992). In addition, a significant decrement of an omega 6 PUFA, eicosatrienoic acid (C20:3n6) was observed, which is consistent with another study on aging (Carver et al., 2001). The levels of PUFAs decrease and MUFAs increase with aging (Lopez et al., 1995). Aberrant lipid metabolism and altered fatty acid composition in the brain have been suggested as an underlying mechanism for pathology involved in brain aging and certain neurodegenerative disorders, including AD and Parkinson’s disease (Cunnane et al., 2012; Fabelo et al., 2011). Some studies reported declined levels of C18:0 (stearic acid), arachidonic acid, and DHA in the brain of mild cognitive impairment and AD, with a significant enhancement of C18:1 (oleic acid), C16:0 (palmitic acid), and VLCFAs (Cunnane et al., 2012; Fraser et al., 2010). It can be concluded that ACOX1 inhibition in the present study may change brain lipid components at a younger age, similar to aging and AD.
Intrinsic membrane properties, categorized into passive and active characteristics of a neuron, can dictate neuronal excitability and affect its input/output gain (Kowalski et al., 2016). DG-GCs in TDYA-treated rats showed a more hyperpolarized RMP with a lower Rin and higher time constant (τm) than the control cells. Hyperpolarized RMP led to neuron hypo-excitability, and may be attributed to changed ion gradient or upregulation of new ion channels active in the resting state (Watari et al., 2013). Rin, and τm are two contributing factors in the integration of postsynaptic signals. Diminution of Rin can result from Rm reduction, which is inversely related to conductivity, and/or enhanced membrane surface. On the other hand, Rm and Cm, the parameters correlative to the cell surface, determine the τm (Young et al., 2009). The granule cells of the TDYA group manifested strongly enhanced τm values compared to the control. Since TDYA influenced Rin and τm in the opposite direction, it can be inferred that Cm was increased by the TDYA administration. Lipid composition can determine this value. In vitro, PUFA application has disclosed their differential effects on membrane capacitance. While eicosapentaenoic acid and arachidonic acid made capacitance increase, docosahexaenoic acid, linoleic acid, oleic acid did not change it (Ong et al., 2006). In analyzing the active properties of the granule cell membrane, no significant difference was observed in some AP parameters, including peak amplitude, threshold, rise time, and decay slope between groups. However, TDYA altered AP properties by enhancing rheobase current, decreasing AP duration, and increasing AHP amplitude with decreased AP firing frequency. The shape of the AP waveform has an essential effect on the firing frequency of a neuron. According to Ohm’s law (R=V/I), decreased Rin in TDYA-treated rats should reduce excitability. Therefore, enhanced rheobase current in this study was predictable. Variations in spike width can influence the gain of a neuron’s input/output relationship (Giese et al., 1998; Lin et al., 2014). Also, the AP duration (width) can be influenced by the rate of spike repolarization, which is commonly regulated by potassium voltage-gated channels (Bean, 2007). For instance, the enhanced density of these channels correlates with declined RMP, Rin, and AP duration observed with maturation for DG-GCs (Spigelman et al., 1992), which is similar to our results. Also, other potassium channels (fast delayed-rectifier K channels or BK channels) have been reported to control the AHP amplitude (Contet et al., 2016; Sesti et al., 2010). Therefore, alteration of potassium channels can be one of the possible mechanisms for ACOX1 inhibition in our study. The effect of fatty acids on ion channels, including potassium, has been subjected to numerous studies. Arachidonic acid, C14:0, C18:1, C18:3n3, and eicosatetraenoic acid have been reported to activate potassium currents. (Ordway et al., 1989). Furthermore, reports show different effects of PUFAs on different potassium channels. For instance, PUFAs have different effects on the voltage-gated potassium channels. While the KV1-4 channels are inactivated in the presence of PUFAs, these lipids can activate the slow rectifier K and BK channels, and increase their currents. (Börjesson & Elinder, 2011; Börjesson et al., 2008; Hoshi et al., 2013; Tian et al., 2016). In addition, both activation and inactivation of channels by PUFAs have been reported in the delayed rectifier potassium channels (Gavrilova-Ruch et al., 2007). It should be mentioned that their double bond, cis-geometry of double bond, and carboxyl group with negative charge have been proposed as the required properties of PUFAs in eliciting these effects (Boland et al., 2009; Elinder & Liin, 2017).
Studies have verified that the brain’s fatty acid components can be influenced by diet, aging, and age-related disorders (Chew et al., 2020; Ledesma et al., 2012; Pakiet et al., 2019). On the other hand, brain lipids are essential determinants of neuron electrical properties. A diet with a low-P/S ratio (supplied 2% of the calories as C18:2n6) affects the electrical properties of dorsal root ganglion cells by reducing AP duration, after hyperpolarization, Rin, and τm compared to a high-P/S diet (supplied 22% of the calories as C18:2n6) (Scott et al., 1989). Another study, focusing on the intrinsic properties of entorhinal cortex cells, indicated that canola/soybean oil in the diet decreased AP duration and postsynaptic response duration and enhanced the firing frequency (Arsenault et al., 2012). The ketogenic diet has displayed an anticonvulsant effect by increasing plasma PUFAs. This effect is mediated by modulating A-type potassium and transient Na channels (Tigerholm et al., 2012). It seems that ACOX1 inhibition may change brain activity by modifying its fatty acid ratios. Finally, according to the results of this study, peroxisomes and hepatic ACOX1 are involved in regulating brain fatty acid profile and electrophysiological properties of DG cells, possibly through potassium channels, which require detailed study in the future.
Ethical Considerations
Compliance with ethical guidelines
The Research and Ethics Committee of the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences approved all experiments (No.: IR.SBMU.MSP.REC.1396.168). Animals were treated according to the guide for the care and use of laboratory animals (National Institutes of Health Publication No. 80-23, revised 1996).
Funding
This article has been extracted from the PhD dissertation of Shahrbanoo Rafiei, approved by School of Medicine Shahid Beheshti University of Medical Sciences (Registration No.: 6). And it is part of the project funded by the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences and Iran National Science Foundation (INSF) (Fund No.: 96015430).
Authors' contributions
Conceptualization, supervision and funding acquisition: Fereshteh Motamedi, Fariba Khodagholi; Methodology: Fereshteh Motamedi, Fariba Khodagholi, Hamid Gholami Pourbadie and Leila Dargahi; Resources: Fereshteh Motamedi, Leila Dargahi and Fariba Khodagholi; Investigation and writing original draft: Shahrbanoo Rafiei; Review & editing: Fereshteh Motamedi, Shahrbanoo Rafiei, Hamid Gholami Pourbadie and Fariba Khodagholi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Shahid Beheshti University of Medical Sciences and Iran National Science Foundation (INSF) for financial support.
References
Arsenault, D., Julien, C., Chen, C. T., Bazinet, R. P., & Calon, F. (2012). Dietary intake of unsaturated fatty acids modulates physiological properties of entorhinal cortex neurons in mice. Journal of Neurochemistry, 122(2), 427-443. [DOI:10.1111/j.1471-4159.2012.07772.x] [PMID]
Arsenault, D., Julien, C., Tremblay, C., & Calon, F. (2011). DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3xTg-AD mice. PloS One, 6(2), e17397. [DOI:10.1371/journal.pone.0017397] [PMID]
Bean, B. P. (2007). The action potential in mammalian central neurons. Nature Reviews. Neuroscience, 8(6), 451-465. [DOI:10.1038/nrn2148] [PMID]
Boland, L. M., Drzewiecki, M. M., Timoney, G., & Casey, E. (2009). Inhibitory effects of polyunsaturated fatty acids on Kv4/KChIP potassium channels. American Journal of Physiology-Cell Physiology, 296(5), C1003-C1014. [DOI:10.1152/ajpcell.00474.2008] [PMID]
Börjesson, S. I., & Elinder, F. (2011). An electrostatic potassium channel opener targeting the final voltage sensor transition. Journal of General Physiology, 137(6), 563-577. [DOI:10.1085/jgp.201110599] [PMID]
Börjesson, S. I., Hammarström, S., & Elinder, F. (2008). Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids. Biophysical Journal, 95(5), 2242-2253. [DOI:10.1529/biophysj.108.130757] [PMID]
Cablé, S., Kedinger, M., & Dauça, M. (1993). Peroxisomes and peroxisomal enzymes along the crypt-villus axis of the rat intestine. Differentiation, 54(2), 99-108. [PMID]
Carver, J. D., Benford, V. J., Han, B., & Cantor, A. B. (2001). The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Research Bulletin, 56(2), 79-85. [DOI:10.1016/S0361-9230(01)00551-2] [PMID]
Chew, H., Solomon, V. A., & Fonteh, A. N. (2020). Involvement of lipids in Alzheimer’s disease pathology and potential therapies. Frontiers in Physiology, 11, 598. [DOI:10.3389/fphys.2020.00598] [PMID]
Cipolla, C. M., & Lodhi, I. J. (2017). Peroxisomal dysfunction in age-related diseases. Trends in Endocrinology & Metabolism, 28(4), 297-308. [DOI:10.1016/j.tem.2016.12.003] [PMID]
Contet, C., Goulding, S. P., Kuljis, D. A., & Barth, A. (2016). BK channels in the central nervous system. International Review of Neurobiology, 128, 281-342. [DOI:10.1016/bs.irn.2016.04.001] [PMID]
Cunnane, S. C., Schneider, J. A., Tangney, C., Tremblay-Mercier, J., Fortier, M., & Bennett, D. A., et al. (2012). Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s Disease, 29(3), 691-697. [DOI:10.3233/JAD-2012-110629] [PMID]
Elinder, F., & Liin, S. I. (2017). Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Frontiers in Physiology, 8, 43. [DOI:10.3389/fphys.2017.00043] [PMID]
Fabelo, N., Martín, V., Santpere, G., Marín, R., Torrent, L., & Ferrer, I., et al. (2011). Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Molecular Medicine, 17(9-10), 1107-1118. [DOI:10.2119/molmed.2011.00119] [PMID]
Ferdinandusse, S., Denis, S., Hogenhout, E. M., Koster, J., van Roermund, C. W., & IJlst, L., et al. (2007). Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Human Mutation, 28(9), 904-912. [DOI:10.1002/humu.20535] [PMID]
Ferdinandusse, S., Denis, S., Mooijer, P. A., Zhang, Z., Reddy, J. K., & Spector, A. A., et al. (2001). Identification of the peroxisomal β-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. Journal of Lipid Research, 42(12), 1987-1995. [DOI:10.1016/S0022-2275(20)31527-3] [PMID]
Fraser, T., Tayler, H., & Love, S. (2010). Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochemical Research, 35(3), 503-513. [DOI:10.1007/s11064-009-0087-5] [PMID]
Gavrilova-Ruch, O., Schönherr, R., & Heinemann, S. H. (2007).Activation of hEAG1 potassium channels by arachidonic acid. Pflugers Archiv : European Journal of Physiology, 453(6), 891-903. [DOI:10.1007/s00424-006-0173-3] [PMID]
Giese, K. P., Storm, J. F., Reuter, D., Fedorov, N. B., Shao, L. R., & Leicher, T., et al. (1998). Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvβ1. 1-deficient mice with impaired learning. Learning & Memory, 5(4), 257-273. [DOI:10.1101/lm.5.4.257]
Hamilton, J. A., Hillard, C. J., Spector, A. A., & Watkins, P. A. (2007). Brain uptake and utilization of fatty acids, lipids and lipoproteins: Application to neurological disorders. Journal of Molecular Neuroscience, 33(1), 2-11. [DOI:10.1007/s12031-007-0060-1] [PMID]
Hiltunen, J. K., Kärki, T., Hassinen, I. E., & Osmundsen, H. (1986). Beta-Oxidation of polyunsaturated fatty acids by rat liver peroxisomes. A role for 2, 4-dienoyl-coenzyme A reductase in peroxisomal beta-oxidation.The Journal of Biological Chemistry, 261(35), 16484–16493. [DOI:10.1016/S0021-9258(18)66592-5] [PMID]
Hoshi, T., Xu, R., Hou, S., Heinemann, S. H., & Tian, Y. (2013). A point mutation in the human Slo1 channel that impairs its sensitivity to omega-3 docosahexaenoic acid. The Journal of General Physiology, 142(5), 507–522. [DOI:10.1085/jgp.201311061] [PMID]
Kowalski, J., Gan, J., Jonas, P., & Pernía-Andrade, A. J. (2016). Intrinsic membrane properties determine hippocampal differential firing pattern in vivo in anesthetized rats. Hippocampus, 26(5), 668-682. [DOI:10.1002/hipo.22550] [PMID]
Ledesma, M. D., Martin, M. G., & Dotti, C. G. (2012). Lipid changes in the aged brain: Effect on synaptic function and neuronal survival. Progress in Lipid Research, 51(1), 23–35. [DOI:10.1016/j.plipres.2011.11.004] [PMID]
Lin, M., Hatcher, J. T., Wurster, R. D., Chen, Q. H., & Cheng, Z. J. (2014). Characteristics of single large-conductance Ca2+-activated K+ channels and their regulation of action potentials and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus. American Journal of Physiology. Cell Physiology, 306(2), C152–C166. [DOI:10.1152/ajpcell.00423.2012] [PMID]
López, G. H., Ilincheta de Boschero, M. G., Castagnet, P. I., & Giusto, N. M. (1995). Age-associated changes in the content and fatty acid composition of brain glycerophospholipids. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 112(2), 331-343. [DOI:10.1016/0305-0491(95)00079-8] [PMID]
Martinez, M. (1992). Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders. Brain Research, 583(1-2), 171-182. [DOI:10.1016/S0006-8993(10)80021-6]
Narayan, V., Ly, T., Pourkarimi, E., Murillo, A. B., Gartner, A., & Lamond, A. I., et al. (2016). Deep proteome analysis identifies age-related processes in C. elegans. Cell Systems, 3(2), 144-159. [DOI:10.1016/j.cels.2016.06.011] [PMID]
Ong, W. L., Jiang, B., Tang, N., Ling, S. F., Yeo, J. F., & Wei, S., et al. (2006). Differential effects of polyunsaturated fatty acids on membrane capacitance and exocytosis in rat pheochromocytoma-12 cells. Neurochemical Research, 31(1), 41-48. [PMID]
Ordway, R. W., Walsh, J. V., Jr, & Singer, J. J. (1989). Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science, 244(4909), 1176-1179. [DOI:10.1126/science.2471269] [PMID]
Pakiet, A., Jakubiak, A., Czumaj, A., Sledzinski, T., & Mika, A. (2019). The effect of western diet on mice brain lipid composition. Nutrition & Metabolism, 16, 81. [DOI:10.1186/s12986-019-0401-4] [PMID]
Poirier, Y., Antonenkov, V. D., Glumoff, T., & Hiltunen, J. K. (2006). Peroxisomal β-oxidation-a metabolic pathway with multiple functions. Biochimica et Biophysica Acta, 1763(12), 1413-1426. [DOI:10.1016/j.bbamcr.2006.08.034] [PMID]
Rafiei, Sh., Khodagholi, F., Motamedi, F., & Dargahi, L. (2021). Peroxisome biogenesis factor 5 (PEX5) controls HDAC6 and SIRT1 expression and modulates mitochondrial biogenesis in rat dorsal hippocampus. Physiology and Pharmacology, 25(4), 341-352 [DOI:10.52547/phypha.25.4.10]
Scott, B., Lew, J., Clandinin, M., & Cinader, B. (1989). Dietary fat influences electric membrane properties of neurons in cell culture. Cellular and Molecular Neurobiology, 9(1), 105-113. [DOI:10.1007/BF00711447] [PMID]
Sesti, F., Liu, S., & Cai, S. Q. (2010). Oxidation of potassium channels by ROS: A general mechanism of aging and neurodegeneration? Trends in Cell Biology, 20(1), 45-51. [DOI:10.1016/j.tcb.2009.09.008] [PMID]
Simons, K., & Toomre, D. (2000). Lipid rafts and signal transduction. Nature Reviews. Molecular Cell Biology, 1(1), 31-39. [DOI:10.1038/35036052] [PMID]
Small, S. A., Chawla, M. K., Buonocore, M., Rapp, P. R., & Barnes, C. A. (2004). Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proceedings of the National Academy of Sciences of the United States of America, 101(18), 7181–7186. [DOI:10.1073/pnas.0400285101] [PMID]
Small, S. A., Tsai, W. Y., DeLaPaz, R., Mayeux, R., & Stern, Y. (2002). Imaging hippocampal function across the human life span: is memory decline normal or not? Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 51(3), 290-295. [DOI:10.1002/ana.10105] [PMID]
Spigelman, I., Zhang, L., & Carlen, P. L. (1992). Patch-clamp study of postnatal development of CA1 neurons in rat hippocampal slices: membrane excitability and K+ currents. Journal of Neurophysiology, 68(1), 55-69. [DOI:10.1152/jn.1992.68.1.55] [PMID]
Tian, Y., Aursnes, M., Hansen, T. V., Tungen, J. E., Galpin, J. D., & Leisle, L., et al. (2016). Atomic determinants of BK channel activation by polyunsaturated fatty acids. Proceedings of the National Academy of Sciences of the United States of America, 113(48), 13905–13910. [DOI:10.1073/pnas.1615562113] [PMID]
Tigerholm, J., Börjesson, S. I., Lundberg, L., Elinder, F., & Fransén, E. (2012). Dampening of hyperexcitability in CA1 pyramidal neurons by polyunsaturated fatty acids acting on voltage-gated ion channels. PloS One, 7(9), e44388. [DOI:10.1371/journal.pone.0044388] [PMID]
Wanders, R. J., & Waterham, H. R. (2006). Biochemistry of mammalian peroxisomes revisited. Annual Review of Biochemistry, 75, 295–332. [DOI:10.1146/annurev.biochem.74.082803.133329] [PMID]
Watari, H., Tose, A. J., & Bosma, M. M. (2013). Hyperpolarization of resting membrane potential causes retraction of spontaneous transients during mouse embryonic circuit development. The Journal of physiology, 591(4), 973-983. [DOI:10.1113/jphysiol.2012.244954] [PMID]
Yang, L., Zhang, Y., Wang, S., Zhang, W., & Shi, R. (2014). Decreased liver peroxisomal β-oxidation accompanied by changes in brain fatty acid composition in aged rats. Neurological Sciences, 35(2), 289-293. [DOI:10.1007/s10072-013-1509-3] [PMID]
Young, C. C., Stegen, M., Bernard, R., Müller, M., Bischofberger, J., & Veh, R. W., et al. (2009). Upregulation of inward rectifier K+ (Kir2) channels in dentate gyrus granule cells in temporal lobe epilepsy. The Journal of Physiology, 587(17), 4213-4233. [DOI:10.1113/jphysiol.2009.170746] [PMID]
Zeng, J., Deng, S., Wang, Y., Li, P., Tang, L., & Pang, Y. (2017). Specific inhibition of Acyl-CoA oxidase-1 by an acetylenic acid improves hepatic lipid and reactive oxygen species (ROS) metabolism in rats fed a high fat diet. Journal of Biological Chemistry, 292(9), 3800-3809. [DOI:10.1074/jbc.M116.763532] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2021/06/20 | Accepted: 2023/06/27 | Published: 2023/09/1
Received: 2021/06/20 | Accepted: 2023/06/27 | Published: 2023/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





 nirp.ir
nirp.ir